8th Grade > Physics
CHEMICAL EFFECTS OF ELECTRIC CURRENT MCQs
Total Questions : 59
| Page 2 of 6 pages
Answer: Option B. -> Zinc
:
B
Rusting of iron takes place in the presence of oxygen, moisture present in the air. A coating of zinc is deposited on the iron to protect it from corrosion and formation of rust. The layer of zinc prevents oxygen from reacting with the iron, making the iron object last much longer.
:
B
Rusting of iron takes place in the presence of oxygen, moisture present in the air. A coating of zinc is deposited on the iron to protect it from corrosion and formation of rust. The layer of zinc prevents oxygen from reacting with the iron, making the iron object last much longer.
Answer: Option A. -> using the impure copper plate as the positive terminal electrode.
:
A
Rohit can purify the copper plate and get the pure copper by using the below mentioned electrolytic setup. On using the impure copper plate as the positive terminal electrode, the metal dissolves from impure copper plate andgoes in the copper sulphate solution.The dissolvedmetal present in the solution is deposited on the cathode and the impurities are left in the copper sulphate solution.
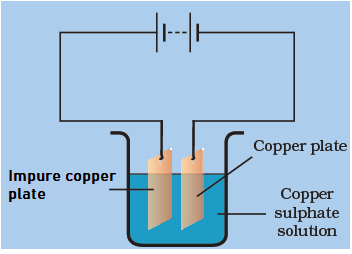
:
A
Rohit can purify the copper plate and get the pure copper by using the below mentioned electrolytic setup. On using the impure copper plate as the positive terminal electrode, the metal dissolves from impure copper plate andgoes in the copper sulphate solution.The dissolvedmetal present in the solution is deposited on the cathode and the impurities are left in the copper sulphate solution.

:
The process is known as electroplating. In this process, electrical energy is converted into chemical energy.
Answer: Option A. -> True
:
A
Electric current, when passed through a salt solution through a specific apparatus, can create a chemical change. Such a process is called Electrolysis. Obtaining a chemical change with the use of electrical energy is known as Chemical Effect of Electricity.
:
A
Electric current, when passed through a salt solution through a specific apparatus, can create a chemical change. Such a process is called Electrolysis. Obtaining a chemical change with the use of electrical energy is known as Chemical Effect of Electricity.
Answer: Option C. -> Chemical energy into electrical energy
:
C
When a cell is connected in a closed circuit, chemical reactions occur inside the cell which results in the release of charge and this charge flows through the circuit as an electric current. Hence, a cell converts chemical energy into electrical energy.
:
C
When a cell is connected in a closed circuit, chemical reactions occur inside the cell which results in the release of charge and this charge flows through the circuit as an electric current. Hence, a cell converts chemical energy into electrical energy.
Answer: Option C. -> Electrolysis
:
C
Electrolysis is the decomposition of an electrolyte when electricity is passed through it. It is a chemical change and results in the formation of new substances.
:
C
Electrolysis is the decomposition of an electrolyte when electricity is passed through it. It is a chemical change and results in the formation of new substances.
Answer: Option B. -> Deposition of metal on the electrodes.
:
B
When an electric current is passed through a solution, positive ions movetowards the electrode which is connected to the negative terminal of the battery. The positive ions take electrons from the negative terminal and may get deposited at the electrode.
:
B
When an electric current is passed through a solution, positive ions movetowards the electrode which is connected to the negative terminal of the battery. The positive ions take electrons from the negative terminal and may get deposited at the electrode.
Answer: Option D. -> Hydrogen gas will be formed at the electrode which is connected to the negative terminal of the battery
:
D
H+ ions move towards the electrode which is connected to the negative terminal of the battery, gain electrons and get released as H2 gas. 2H++2e−→H2
:
D
H+ ions move towards the electrode which is connected to the negative terminal of the battery, gain electrons and get released as H2 gas. 2H++2e−→H2
Answer: Option A. -> magnetic needle deflects more in the case of sea water.
:
A
Sea water is more conductingcompared to drinking water, as the former has more salts. So, more current flows through sea water than through drinking water. Hence, when tested with a tester, the magnetic deflection will be more in the case of sea water.
:
A
Sea water is more conductingcompared to drinking water, as the former has more salts. So, more current flows through sea water than through drinking water. Hence, when tested with a tester, the magnetic deflection will be more in the case of sea water.
Answer: Option B. -> Distilled water
:
B
Distilled water does not have any ions for the conduction of electricity, while salt water, tap water, and ORS drink have salts present in them which form ions in water and conduct electricity. Hence, distilled water is a bad conductor of electricity.
:
B
Distilled water does not have any ions for the conduction of electricity, while salt water, tap water, and ORS drink have salts present in them which form ions in water and conduct electricity. Hence, distilled water is a bad conductor of electricity.
















