Question
Rohit has an impure copper plate and wants to purify it by electroplating. Using an electrolytic setup, he can do this by
Answer: Option A
:
A
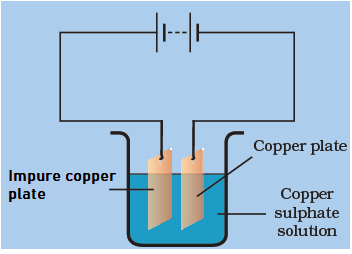
Rohit can purify the copper plate and get the pure copper by using the below mentioned electrolytic setup. On using the impure copper plate as the positive terminal electrode, the metal dissolves from impure copper plate andgoes in the copper sulphate solution.The dissolvedmetal present in the solution is deposited on the cathode and the impurities are left in the copper sulphate solution.
Was this answer helpful ?
:
A
Rohit can purify the copper plate and get the pure copper by using the below mentioned electrolytic setup. On using the impure copper plate as the positive terminal electrode, the metal dissolves from impure copper plate andgoes in the copper sulphate solution.The dissolvedmetal present in the solution is deposited on the cathode and the impurities are left in the copper sulphate solution.

Was this answer helpful ?


















Submit Solution