8th Grade > Physics
CHEMICAL EFFECTS OF ELECTRIC CURRENT MCQs
:
C
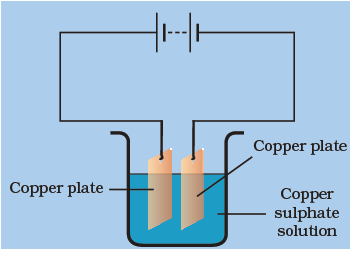
If we replace the copper plate which was connected to the negative terminal of the battery with a carbon rod, we obtain a coating of copper on the carbon rod. The copper ions from the solution (copper sulphate) get deposited on the electrodes (carbon rods).
:
A
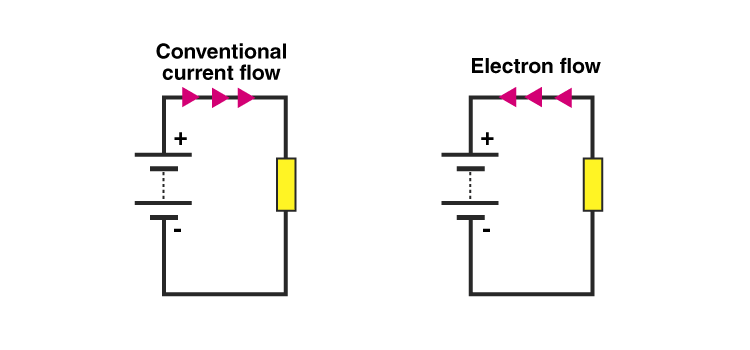
In an electric circuit, the flow of electrons takes place from the negative terminal to the positive terminal. The conventional flow of electric current is taken in the direction of positive charge flow i.e., in the opposite direction to the flow of electrons. It means the conventional direction of electric current is from the positive terminal to the negative terminal of the circuit, as shown in the diagram above.
:
B
Tin cans used for storing food are made by electroplating tin onto iron. Tin is less reactive than iron. Thus, food does not come into contact with iron and is protected from getting spoilt.
:
C
A solution conducts electricity if it contains ions. Ions have the ability to receive or donate electrons and hence acts as charge carriers within a liquid solution.
:
A
The given statement is true. The current carrying wire creates a magnetic field around itself which causes the magnetic needle to deflect from its usual north-south direction.
:
D
For electroplating, the electrolytic cell consists of two electrodes held apart from one another and immersed in an electrolyte. But for this to occur, the electrodes need to be conducting.
Sandeep cannot electroplate his pencil because the pencil is made of wood which is a non-conducting material. So, it cannot act as an electrode.
:
B
The objects which allow electric currents to flow through them easily are known as good conductors of electricity.
:
C
Metals are good conductors of electricity because they have free electrons. These free electrons act as charge carriers in the metallic structure, allowing electric current to flow through them.
:
A
Metals contain free electrons which start drifting on applying a voltage. Electric current in a metallic wire is due to flow of these negatively charged electrons.