8th Grade > Physics
CHEMICAL EFFECTS OF ELECTRIC CURRENT MCQs
Total Questions : 59
| Page 1 of 6 pages
Answer: Option C. -> Light Emitting Diode
:
C
LED stands for Light Emitting Diode. Compared to an electric bulb, it requires less current for it to glow. It is used in a tester to check the conductivity of the liquid.
:
C
LED stands for Light Emitting Diode. Compared to an electric bulb, it requires less current for it to glow. It is used in a tester to check the conductivity of the liquid.
Answer: Option B. -> less
:
B
LED needs less electric current to glow compared to the normal bulb. LED does not need to burn filament to produce light. Hence, LED-based tester is better for testing conductivity of liquids (like salt solutions) with low electrical conductivity.
:
B
LED needs less electric current to glow compared to the normal bulb. LED does not need to burn filament to produce light. Hence, LED-based tester is better for testing conductivity of liquids (like salt solutions) with low electrical conductivity.
Answer: Option A. -> True
:
A
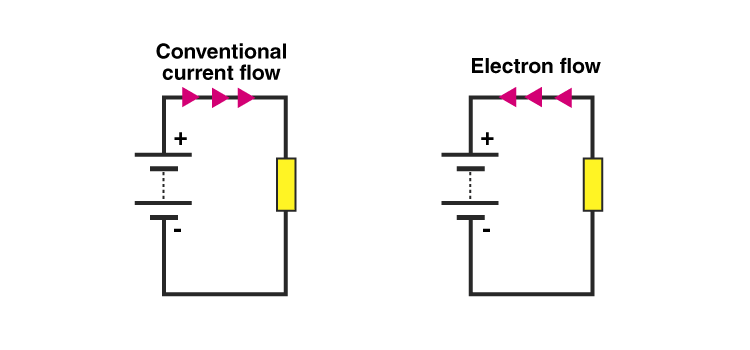
In an electric circuit, the flow of electrons takes place from the negative terminal to the positive terminal. The conventional flow of electric current is taken in the direction of positive charge flow i.e., in the opposite direction to the flow of electrons. It means the conventional direction of electric current is from the positive terminal to the negative terminal of the circuit, as shown in the diagram above.
:
A

In an electric circuit, the flow of electrons takes place from the negative terminal to the positive terminal. The conventional flow of electric current is taken in the direction of positive charge flow i.e., in the opposite direction to the flow of electrons. It means the conventional direction of electric current is from the positive terminal to the negative terminal of the circuit, as shown in the diagram above.
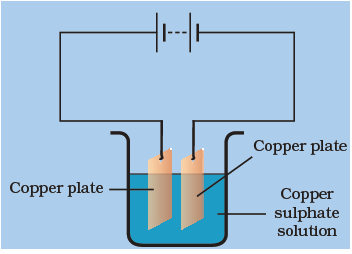
Answer: Option C. -> Copper gets deposited on the electrode connected to the negative terminal of the battery.
:
C
When electric current is passed through the copper sulphate solution, copper sulphate dissociates into Cu2+ and SO2−4 ions. The Cu2+ get drawn to the electrode connected to the negative terminal of the battery and gets deposited on it.
:
C
When electric current is passed through the copper sulphate solution, copper sulphate dissociates into Cu2+ and SO2−4 ions. The Cu2+ get drawn to the electrode connected to the negative terminal of the battery and gets deposited on it.
Answer: Option C. -> Heating effect of current
:
C
The electric current in a metal wire is nothing but the flow of electrons. When these electrons pass throughthe filament of the bulb, it produces heat and thereby light energy. Hence, this heating effect of electric current is responsible for the glow.
:
C
The electric current in a metal wire is nothing but the flow of electrons. When these electrons pass throughthe filament of the bulb, it produces heat and thereby light energy. Hence, this heating effect of electric current is responsible for the glow.
Answer: Option A. -> True
:
A
When two or more cells are connected together end-to-end, it makes a battery. The typical advantage of connecting cells in a battery is to add the voltage of all the cells together to get a higher voltage.
:
A
When two or more cells are connected together end-to-end, it makes a battery. The typical advantage of connecting cells in a battery is to add the voltage of all the cells together to get a higher voltage.
Answer: Option C. -> Dilute hydrochloric acid conducts electricity
:
C
Pure water that is completely free of all salts is a bad conductor of electricity. Distilled water is an example. To make it conducting, it should have ions to carry charges. Substances like salts, acidsand baseswhen dissolved in water dissociate toform ions. So, they conduct electricity.
:
C
Pure water that is completely free of all salts is a bad conductor of electricity. Distilled water is an example. To make it conducting, it should have ions to carry charges. Substances like salts, acidsand baseswhen dissolved in water dissociate toform ions. So, they conduct electricity.
Answer: Option A. -> True
:
A
A material is said to be a good conductor if it allows electricity to pass through it easily. Materials which are classified as poor conductors does not also allow electricity to pass through them easily. However, if the electrical source is strong enough, it may allow to pass electric current through it. For example, air is a poor conductor of electricity. However, during lightning, air allows the electric current to pass through it.
:
A
A material is said to be a good conductor if it allows electricity to pass through it easily. Materials which are classified as poor conductors does not also allow electricity to pass through them easily. However, if the electrical source is strong enough, it may allow to pass electric current through it. For example, air is a poor conductor of electricity. However, during lightning, air allows the electric current to pass through it.
Answer: Option C. -> Cells of the battery drain very quickly.
:
C
When the circuit is closed, charges start flowing from one end to the other. While checking the LED, the circuit should not be closed for a long time. Otherwise, the cells of the battery will drain very quickly as this offers very little resistance to the flow of current.
:
C
When the circuit is closed, charges start flowing from one end to the other. While checking the LED, the circuit should not be closed for a long time. Otherwise, the cells of the battery will drain very quickly as this offers very little resistance to the flow of current.
Answer: Option B. -> False
:
B
A solution can conduct electricity when it has ions which act as charge carriers. When sugar is added to water, it does not produce ions to facilitate electrical conductivity. On the other hand, asalt solution has ions to conduct electricity. So, a sugar solution is apoor conductor as compared to a salt solution.
:
B
A solution can conduct electricity when it has ions which act as charge carriers. When sugar is added to water, it does not produce ions to facilitate electrical conductivity. On the other hand, asalt solution has ions to conduct electricity. So, a sugar solution is apoor conductor as compared to a salt solution.