8th Grade > Physics
CHEMICAL EFFECTS OF ELECTRIC CURRENT MCQs
Total Questions : 59
| Page 3 of 6 pages
Answer: Option D. -> Cannot be done as wood is a non-conducting material.
:
D
For electroplating, the electrolytic cell consists of twoelectrodes held apart from one another and immersed in an electrolyte. But for thisto occur, the electrodes need to be conducting.
Sandeep cannot electroplate his pencil because the pencil is made of wood which is a non-conducting material. So, it cannot act as an electrode.
:
D
For electroplating, the electrolytic cell consists of twoelectrodes held apart from one another and immersed in an electrolyte. But for thisto occur, the electrodes need to be conducting.
Sandeep cannot electroplate his pencil because the pencil is made of wood which is a non-conducting material. So, it cannot act as an electrode.
Answer: Option A. -> True
:
A
The given statement is true.The current carrying wire creates a magnetic field around itself which causes the magnetic needle to deflect from its usual north-south direction.
:
A
The given statement is true.The current carrying wire creates a magnetic field around itself which causes the magnetic needle to deflect from its usual north-south direction.
Answer: Option A. -> electrons
:
A
Metals contain free electrons which start drifting on applying a voltage. Electric current in a metallic wire is due to flow of these negatively charged electrons.
:
A
Metals contain free electrons which start drifting on applying a voltage. Electric current in a metallic wire is due to flow of these negatively charged electrons.
Answer: Option B. -> Good conductors
:
B
The objects which allow electric currents to flow through them easily are known as good conductors of electricity.
:
B
The objects which allow electric currents to flow through them easily are known as good conductors of electricity.
Answer: Option C. -> Copper gets coated on the carbon rod.
:
C
If we replace the copper plate which was connected to the negative terminal of the battery with a carbon rod, we obtain a coating of copper on the carbon rod. The copper ions from the solution (copper sulphate) get deposited on the electrodes (carbon rods).
:
C
If we replace the copper plate which was connected to the negative terminal of the battery with a carbon rod, we obtain a coating of copper on the carbon rod. The copper ions from the solution (copper sulphate) get deposited on the electrodes (carbon rods).
Answer: Option A. -> Common salt
:
A
Pure water that is completely free of all salts is a bad conductor of electricity. To make it conducting, it should have ions to carry charges. Salts, when dissolved in water, can produce ions that can carry electricity. The chemical formula of common salt is NaCl (Sodium Chloride). It dissociates into sodium ion and chloride ion which act like charge carriers to conduct electricity.
Sugar, glucose, and kerosene do not form ions in water and cannot conduct electricity.
:
A
Pure water that is completely free of all salts is a bad conductor of electricity. To make it conducting, it should have ions to carry charges. Salts, when dissolved in water, can produce ions that can carry electricity. The chemical formula of common salt is NaCl (Sodium Chloride). It dissociates into sodium ion and chloride ion which act like charge carriers to conduct electricity.
Sugar, glucose, and kerosene do not form ions in water and cannot conduct electricity.
Answer: Option B. -> Food does not get spoilt as it does not come in contact with iron which is more reactive.
:
B
Tin cans used for storing food are made by electroplating tin onto iron. Tin is less reactive than iron. Thus, food does not come into contact with iron and is protected from getting spoilt.
:
B
Tin cans used for storing food are made by electroplating tin onto iron. Tin is less reactive than iron. Thus, food does not come into contact with iron and is protected from getting spoilt.
Answer: Option C. -> Free electrons
:
C
Metals are good conductors of electricity because they have free electrons. These free electrons act as charge carriers in the metallic structure, allowing electric current to flow through them.
:
C
Metals are good conductors of electricity because they have free electrons. These free electrons act as charge carriers in the metallic structure, allowing electric current to flow through them.
Answer: Option C. -> The solution consists of ions.
:
C
A solution conducts electricity if it contains ions. Ions have the ability to receive or donate electrons and hence acts as charge carriers within a liquid solution.
:
C
A solution conducts electricity if it contains ions. Ions have the ability to receive or donate electrons and hence acts as charge carriers within a liquid solution.
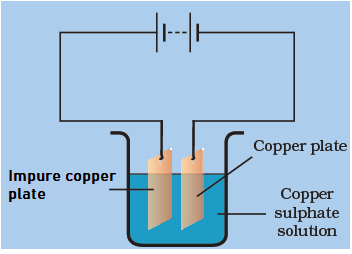
Answer: Option A. ->
using the impure copper plate as the positive terminal electrode.
:
A
:
A
Rohit can purify the copper plate and get the pure copper by using the below mentioned electrolytic setup. On using the impure copper plate as the positive terminal electrode, the metal dissolves from impure copper plate and goes in the copper sulphate solution. The dissolved metal present in the solution is deposited on the cathode and the impurities are left in the copper sulphate solution.