10th Grade > Chemistry
PERIODIC CLASSIFICATION OF ELEMENTS MCQs
Total Questions : 54
| Page 5 of 6 pages
Answer: Option B. ->
46u
:
B
:
B
The molecular formula of carbon dioxide is CO2.
Molecular mass of CO2 made from 146C=14+(16+16)=46 u.
Answer: Option A. ->
A, B, C
:
A
:
A
The drawbacks of Newland's law of octaves are -
- The law of octaves was found to be applicable only till calcium. It was not applicable to elements of higher atomic masses.
- The position of hydrogen along with fluorine and chlorine was not justified on the basis of chemical properties.
- Newland placed two elements in the same slot to fit elements in the table. He also placed some unlike elements under the same group.
- For example, cobalt (Co) and nickel (Ni) are placed in the same group as that of fluorine, chlorine and bromine. But cobalt and nickel have properties quite different from fluorine, chlorine and bromine. Similarly, iron which has resemblances with cobalt and nickel in its properties has been placed far away from these elements.
Answer: Option A. ->
Oxides
:
A and C
:
A and C
Mendeleev considered oxides and hydrides of elements for classifying their properties. This is because oxygen and hydrogen reacts with most of the elements to form compounds due to their high reactivity.
Answer: Option C. ->
Hydrogen
:
C
Hydrogen can gain one electron and can become H− ion just like halogens (group 17) form X− ion. Also, it can lose an electron and can become H+ ion just like alkali metals (group 1) form M+ ion. Hence, the position of hydrogen was controversial in the modern periodic table although it is considered as a non-metal.
:
C
Hydrogen can gain one electron and can become H− ion just like halogens (group 17) form X− ion. Also, it can lose an electron and can become H+ ion just like alkali metals (group 1) form M+ ion. Hence, the position of hydrogen was controversial in the modern periodic table although it is considered as a non-metal.
Answer: Option A. ->
Beryllium
:
A
The atomic size of an atom is the distance between the nucleus and the outermost shell of an atom. Atomic size reduces across a period from left to right. Beryllium, carbon, oxygen and fluorine belong to second period. Since beryllium is placed at the leftmost side of the period, therefore it has the largest atomic size.
:
A
The atomic size of an atom is the distance between the nucleus and the outermost shell of an atom. Atomic size reduces across a period from left to right. Beryllium, carbon, oxygen and fluorine belong to second period. Since beryllium is placed at the leftmost side of the period, therefore it has the largest atomic size.
Answer: Option C. ->
Germanium
:
C
Mendeleev predicted the existence of few elements and left empty spaces in his periodic table. One such element whose existence he predicted was eka-silicon. In the modern periodic table, germanium has the properties similar to that of eka-silicon.
:
C
Mendeleev predicted the existence of few elements and left empty spaces in his periodic table. One such element whose existence he predicted was eka-silicon. In the modern periodic table, germanium has the properties similar to that of eka-silicon.
Answer: Option A. ->
Isobars
:
A
:
A
Since the given elements have same mass numbers but different atomic numbers, they are isobars.
Answer: Option B. ->
14
:
B
:
B
Mass number = No. of protons + No. of neutrons
Since an atom is neutral, no. of protons = no. of electrons, which is necessary for charge balance.
Given, the number of electrons = 13. Therefore, the number of protons is 13.
So, number of neutrons will be 27 – 13 = 14
Answer: Option D. ->
I
:
D
:
D
Iodine is a lustrous non-metal.
Answer: Option D. ->
Isotopes take the same place as the element.
:
A, B, C, and D
In the modern periodic table given below, we can see that all the elements are arranged in the increasing order of their atomic numbers.
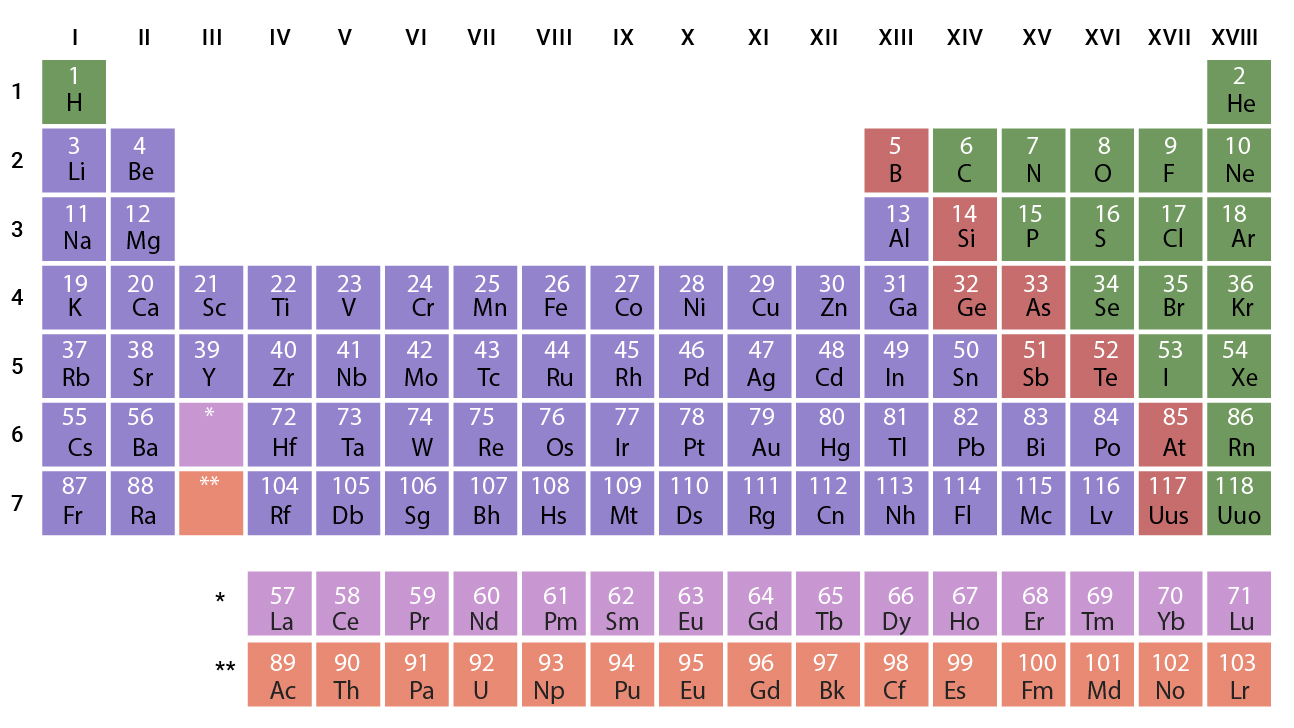
Carbon, for an example, has many isotopes and allotropes. But even so, there is only one place for carbon. Thus, the remaining statements are also true. Also, metals are placed on the left side, non-metals on the right side, and metalloids in between them in a zig-zag manner.
:
A, B, C, and D
In the modern periodic table given below, we can see that all the elements are arranged in the increasing order of their atomic numbers.

Carbon, for an example, has many isotopes and allotropes. But even so, there is only one place for carbon. Thus, the remaining statements are also true. Also, metals are placed on the left side, non-metals on the right side, and metalloids in between them in a zig-zag manner.
















