10th Grade > Chemistry
PERIODIC CLASSIFICATION OF ELEMENTS MCQs
:
B
Elements, when arranged in order of increasing atomic number, lead us to the classification known as the modern periodic table. The chemical and physical properties of the elements vary periodically when the elements are arranged in the order of their atomic numbers.
:
B
Alkali metals are the elements in the 1st group of the periodic table other than hydrogen. The first alkali metal in the modern periodic table is lithium (Li).
:
C
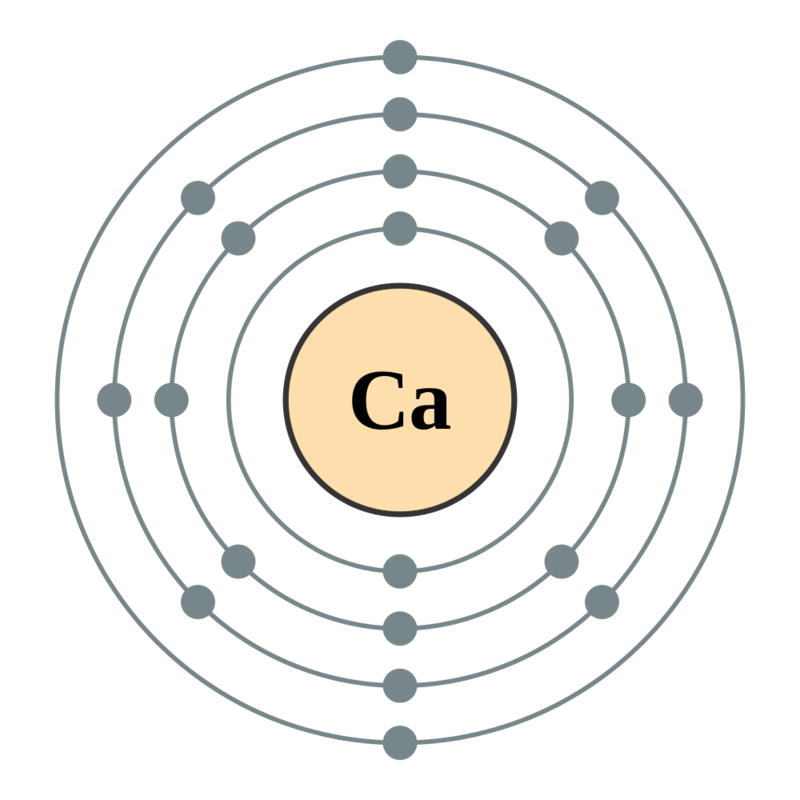
The number of shells in calcium can be determined by writing its electronic configuration. Calcium has an atomic number of 20, which means it has 20 electrons. There are 2 electrons in the first shell, 8 electrons in the second shell, another 8 electrons in the third shell, and 2 in the fourth (outermost) shell of calcium.
Its electronic configuration can be written as 2, 8, 8, 2. The number of shells in calcium which are filled with electrons are 4.
:
A and C
In the modern periodic table, there are 18 groups and 7 periods. Elements are arranged in rows and columns. The horizontal rows are known as periods while the vertical columns are known as groups.
:
A
The elements in the 1st group of the periodic table have only one valence electron. Thus, they will tend to lose only one electron to gain the the nearest noble gas configuration.
So, valency of the elements in the 1st group of the modern periodic table is 1 and they will lose one electron to form unipositively charged ion, (A+).
:
C
Valency is the combining capacity of the element.
Nitrogen has atomic number 7. Electronic configuration of nitrogen can be written as:
N=1s2,2s2,2p3
It has 5 electrons in its outermost shell and requires 3 more electrons in order to complete its L shell. Hence its valency is 3.
:
D
- Dobereiner arranged elements in groups of three, called triads, in the increasing order of their atomic masses. In a triad, the atomic mass of the middle element was roughly equal to the average of the atomic masses of the other two elements.
- From the given options, the only element that can form a Dobereiner's Triad, along with lithium and sodium is only pottassium.
- If A, B and C are Dobereiner's Triads, Atomic mass of B=Atomic mass of A+Atomic mass of C2
:
B
When Mendeleev made the periodic table the number of elements discovered till then were 63. Rest were discovered later, and were added consecutively.