10th Grade > Chemistry
PERIODIC CLASSIFICATION OF ELEMENTS MCQs
Total Questions : 54
| Page 1 of 6 pages
Answer: Option C. -> 3
:
C
Valency is the combining capacity of the element.
Nitrogen has atomic number 7. Electronic configuration of nitrogen can be written as:
N=1s2,2s2,2p3
It has 5 electrons in its outermost shell andrequires 3 more electrons in order to complete its L shell.Hence its valency is 3.
:
C
Valency is the combining capacity of the element.
Nitrogen has atomic number 7. Electronic configuration of nitrogen can be written as:
N=1s2,2s2,2p3
It has 5 electrons in its outermost shell andrequires 3 more electrons in order to complete its L shell.Hence its valency is 3.
Answer: Option C. -> Hydrogen
:
C
Hydrogen can gain one electron and can become H− ion just like halogens (group 17) form X− ion. Also, it can lose an electron and can become H+ ion just like alkali metals (group 1) form M+ ion. Hence, the position of hydrogen was controversial in the modern periodic table although it is considered as a non-metal.
:
C
Hydrogen can gain one electron and can become H− ion just like halogens (group 17) form X− ion. Also, it can lose an electron and can become H+ ion just like alkali metals (group 1) form M+ ion. Hence, the position of hydrogen was controversial in the modern periodic table although it is considered as a non-metal.
Answer: Option A. -> A, B, C
:
A
The drawbacks of Newland's law of octaves are -
:
A
The drawbacks of Newland's law of octaves are -
- The law of octaves was found to beapplicable only till calcium.It was not applicable toelements of higher atomic masses.
- The position of hydrogen along with fluorine and chlorine was not justified on the basis of chemicalproperties.
- Newlandplaced two elements in the same slotto fit elements in the table. He alsoplacedsome unlike elementsunder the same group.
- For example, cobalt (Co) and nickel (Ni) are placed in the samegroup as that offluorine, chlorine and bromine. But cobalt and nickel have properties quitedifferent from fluorine, chlorine and bromine. Similarly, iron which has resemblances with cobalt andnickel in its properties has been placed far away from these elements.
Answer: Option C. -> Four
:
C
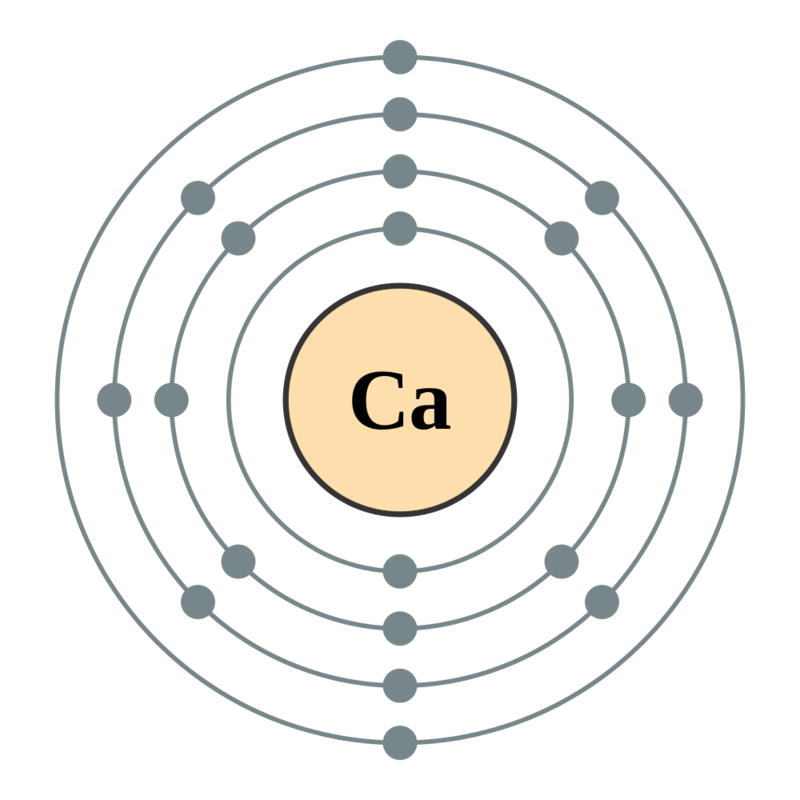
The number of shells in calcium can be determined by writing its electronic configuration. Calcium has an atomic number of 20, which means it has 20 electrons. There are 2 electrons in the firstshell, 8 electrons in the second shell, another 8 electrons in the thirdshell, and 2 in the fourth (outermost) shell of calcium.
Its electronic configuration can be written as 2, 8,8, 2. The number of shells in calcium which are filled with electrons are 4.
:
C

The number of shells in calcium can be determined by writing its electronic configuration. Calcium has an atomic number of 20, which means it has 20 electrons. There are 2 electrons in the firstshell, 8 electrons in the second shell, another 8 electrons in the thirdshell, and 2 in the fourth (outermost) shell of calcium.
Its electronic configuration can be written as 2, 8,8, 2. The number of shells in calcium which are filled with electrons are 4.
Answer: Option A. -> increases
:
A
Metallic character is the tendency of an element to lose an electron easily. As we go down the group, the size of atoms increases.
Due to this, the nuclear force of attraction on the electrons in the outermost shell decreases, because of which they can be removed easily. Therefore, metallic character increases down the group.
:
A
Metallic character is the tendency of an element to lose an electron easily. As we go down the group, the size of atoms increases.
Due to this, the nuclear force of attraction on the electrons in the outermost shell decreases, because of which they can be removed easily. Therefore, metallic character increases down the group.
Answer: Option C. -> 30
:
C
According to Dobereiner's law of triads, the atomic mass of the middle element of the triad is equal to the arithmetic mean of the atomic masses of the other two elements.
Since, Y is the middle element of the triad, its mass should be the average of X and Z, that is,
Y=X+Z2=20+402=30u
:
C
According to Dobereiner's law of triads, the atomic mass of the middle element of the triad is equal to the arithmetic mean of the atomic masses of the other two elements.
Since, Y is the middle element of the triad, its mass should be the average of X and Z, that is,
Y=X+Z2=20+402=30u
Answer: Option A. -> Isobars
:
A
Since the given elements have same mass numbers but different atomic numbers, they are isobars.
:
A
Since the given elements have same mass numbers but different atomic numbers, they are isobars.
Answer: Option B. -> 14
:
B
Mass number = No. of protons + No. of neutrons
Since an atom is neutral, no. of protons = no. of electrons, which is necessary for charge balance.
Given, the number of electrons = 13. Therefore, the number of protons is 13.
So, number of neutrons will be 27 – 13 = 14
:
B
Mass number = No. of protons + No. of neutrons
Since an atom is neutral, no. of protons = no. of electrons, which is necessary for charge balance.
Given, the number of electrons = 13. Therefore, the number of protons is 13.
So, number of neutrons will be 27 – 13 = 14
Answer: Option D. -> Potassium, atomic mass = 39 u
:
D
:
D
- Dobereiner arranged elements in groups of three, called triads,in the increasing order of their atomic masses. In a triad, the atomic mass of the middle element was roughly equal to the average of the atomic masses of the other two elements.
- From the given options, the only element that can form aDobereiner's Triad, along with lithium and sodiumis only pottassium.
- If A, B and C are Dobereiner's Triads, Atomic mass of B=Atomic mass of A+Atomic mass of C2