Question
How many shells in calcium atom are filled with electrons?
Answer: Option C
:
C
Was this answer helpful ?
:
C
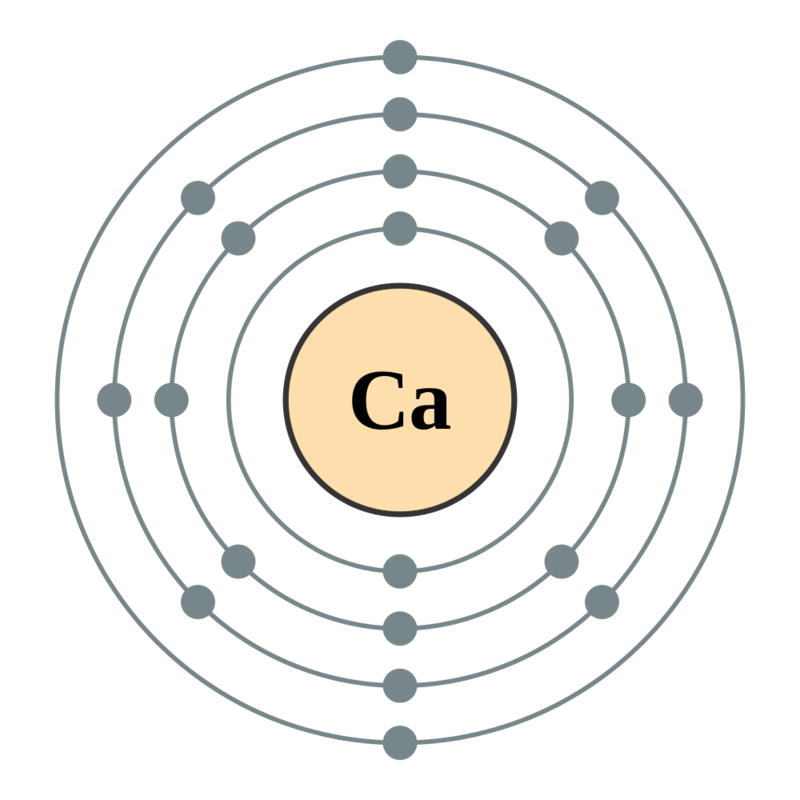
The number of shells in calcium can be determined by writing its electronic configuration. Calcium has an atomic number of 20, which means it has 20 electrons. There are 2 electrons in the first shell, 8 electrons in the second shell, another 8 electrons in the third shell, and 2 in the fourth (outermost) shell of calcium.
Its electronic configuration can be written as 2, 8, 8, 2. The number of shells in calcium which are filled with electrons are 4.
Was this answer helpful ?
More Questions on This Topic :
Question 1.
In the modern periodic table, there are ___
....
Question 4.
The valency of nitrogen in N2 molecule is:
....
Question 5. Isotopes differ in:....


















Submit Solution