12th Grade > Chemistry
ALCOHOLS PHENOLS AND ETHERS MCQs
Total Questions : 28
| Page 2 of 3 pages
Question 11. Ortho-nitrophenol is steam volatile whereas para-nitrophenol is not. This is due to
1.Intramolecular hydrogen bonding present in ortho-nitrophenol
2.Intermolecular hydrogen bonding present in para-nitrophenol
3.Intramolecular hydrogen bonding present in para-nitrophenol
4.Inter-molecular hydrogen bonding present in ortho-nitrophenol.
1.Intramolecular hydrogen bonding present in ortho-nitrophenol
2.Intermolecular hydrogen bonding present in para-nitrophenol
3.Intramolecular hydrogen bonding present in para-nitrophenol
4.Inter-molecular hydrogen bonding present in ortho-nitrophenol.
Answer: Option C. -> 1&2
:
C
Para-nitro phenol has higher boiling point than ortho-nitrophenol due to intermolecular hydrogen bonding present in para-nitrophenol, which requires more energy to break these bonds during boiling. In o-nitrophenol, intramolecular hydrogen bonding is present to greater extent. Thus,o-nitrophenolis steam volatile due to low boiling point.
:
C
Para-nitro phenol has higher boiling point than ortho-nitrophenol due to intermolecular hydrogen bonding present in para-nitrophenol, which requires more energy to break these bonds during boiling. In o-nitrophenol, intramolecular hydrogen bonding is present to greater extent. Thus,o-nitrophenolis steam volatile due to low boiling point.
Answer: Option C. -> 1&2
:
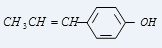
B
Reaction proceeds by benzyl carbocation formation which is stabilized due to resonance
:
B
Reaction proceeds by benzyl carbocation formation which is stabilized due to resonance
Answer: Option C. -> ethanol
:
C
Order of Acidity−o−nitrophenol>phenol>o−cresol>ethanol. o−nitrophenol is more acidic than phenol due to electron withdrawing nature of nitro group. o-crseol is less acidic than alcohol due to electron-releasing nature of methyl group. Phenoxide ion is resonance stabilized but ethoxide ion is not. Thus, phenol is more acidic than alcohol. Greater the pKavalue, less is the acidity.
:
C
Order of Acidity−o−nitrophenol>phenol>o−cresol>ethanol. o−nitrophenol is more acidic than phenol due to electron withdrawing nature of nitro group. o-crseol is less acidic than alcohol due to electron-releasing nature of methyl group. Phenoxide ion is resonance stabilized but ethoxide ion is not. Thus, phenol is more acidic than alcohol. Greater the pKavalue, less is the acidity.
Answer: Option D. -> Butanone
:
D
Diethyl ether is C4H10O. n-propylmethylether, butan-1-ol & 2-methyl~propan-2-ol is C4H10O. Butanone is C4H8O.
:
D
Diethyl ether is C4H10O. n-propylmethylether, butan-1-ol & 2-methyl~propan-2-ol is C4H10O. Butanone is C4H8O.
Answer: Option A. -> Ethanol is obtained by fermentation of sugar in presence of enzyme zymase which converts to glucose and glucose is followed by fermentation with enzyme invertase.
:
A
Ethanol is obtained by fermentation of sugar in presence of enzyme invertase which is converted to glucose and glucose is then treated with enzymezymase.
:
A
Ethanol is obtained by fermentation of sugar in presence of enzyme invertase which is converted to glucose and glucose is then treated with enzymezymase.
Answer: Option A. -> Williamson's synthesis proceeds by SN2 attack of a nucleophile.
:
A
Acidic dehydration of primary alcohols at low temperature gives ethers due to less steric hindrance. The reaction follows SN1 pathway when the alcohol is secondary or tertiary. Reaction of sodium tertiary butoxide with methyl bromide gives t-butyl methyl ether, as the alkyl halide is primary andunhindered Boiling point of ether is less than that of primary alcohol due to absence of hydrogen bonding in ether.
:
A
Acidic dehydration of primary alcohols at low temperature gives ethers due to less steric hindrance. The reaction follows SN1 pathway when the alcohol is secondary or tertiary. Reaction of sodium tertiary butoxide with methyl bromide gives t-butyl methyl ether, as the alkyl halide is primary andunhindered Boiling point of ether is less than that of primary alcohol due to absence of hydrogen bonding in ether.
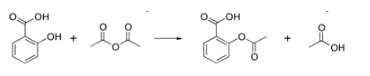
Answer: Option B. -> Acetylation of salicylic acid gives aspirin
:
B
Since methoxy group in anisole is activating and o & p-directing, major product is 2-nitro anisole & minor product is 4-nitro anisole.
Acetylation of salicylic acid gives aspirin.
Reimer-Tiemann reaction in presence of CHCl3&aq.NaOH with phenol leads to attack of electrophile +CHCl2 on sp2hybridised carbon in phenoxide ion.
Phenol is commercially prepared from oxidation of cumene followed by treatment with acid. This reaction leads phenol & acetone as by-product in large quantities. Dow’s process involves treatment of Chlorobenzene with fused NaOH under high pressure conditions followed by acidification.
:
B
Since methoxy group in anisole is activating and o & p-directing, major product is 2-nitro anisole & minor product is 4-nitro anisole.
Acetylation of salicylic acid gives aspirin.

Reimer-Tiemann reaction in presence of CHCl3&aq.NaOH with phenol leads to attack of electrophile +CHCl2 on sp2hybridised carbon in phenoxide ion.
Phenol is commercially prepared from oxidation of cumene followed by treatment with acid. This reaction leads phenol & acetone as by-product in large quantities. Dow’s process involves treatment of Chlorobenzene with fused NaOH under high pressure conditions followed by acidification.
Answer: Option C. -> Acetylene
:
C
Ethanol in presence of conc. Sulphuric acid gives ethylene at 170∘C.
At lower temperature of 140∘C it gives diethyl ether.
At 70∘C−110∘C ethanol dehydrates to give ethyl hydrogen sulphate.
:
C
Ethanol in presence of conc. Sulphuric acid gives ethylene at 170∘C.
At lower temperature of 140∘C it gives diethyl ether.
At 70∘C−110∘C ethanol dehydrates to give ethyl hydrogen sulphate.