12th Grade > Physics
KINETIC THEORY OF GASES MCQs
Total Questions : 24
| Page 3 of 3 pages
Answer: Option C. -> VRT(P − P′)
:
C
Number of moles present initially is n=PVRT. Let n′ be the number of moles of the gas that leaked till
the pressure falls to P′. Since volume V of the vessel cannot change and temperature T remains
constant during leakage, we have
n′=P′VRT
∴Number of moles that leaked is
Δn=n−n′=PVRT−P′VRT=VRT(P−P′)
So the correct choice is (C)
:
C
Number of moles present initially is n=PVRT. Let n′ be the number of moles of the gas that leaked till
the pressure falls to P′. Since volume V of the vessel cannot change and temperature T remains
constant during leakage, we have
n′=P′VRT
∴Number of moles that leaked is
Δn=n−n′=PVRT−P′VRT=VRT(P−P′)
So the correct choice is (C)
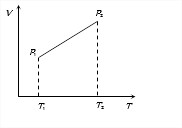
Answer: Option C. -> P1
:
C
In case of given graph, V and T are related as V = aT +b, where a and b are constants.
From ideal gas equation, PV = μRT
We find P = μRTaT+b = μa+bT
Since T2>T1, therefore P2>P1.
:
C
In case of given graph, V and T are related as V = aT +b, where a and b are constants.
From ideal gas equation, PV = μRT
We find P = μRTaT+b = μa+bT
Since T2>T1, therefore P2>P1.
Answer: Option B. -> 126 J
:
B
(△Q)P = μCP△T and (△Q)V = μCV△T
⇒ (△Q)V(△Q)P = CvCp = 32R52R = 35
[∵ (CV)mono=32R,(CP)mono=52R]
⇒ (△Q)V = 35×(△Q)P = 35×210=126J
:
B
(△Q)P = μCP△T and (△Q)V = μCV△T
⇒ (△Q)V(△Q)P = CvCp = 32R52R = 35
[∵ (CV)mono=32R,(CP)mono=52R]
⇒ (△Q)V = 35×(△Q)P = 35×210=126J
Answer: Option D. -> 150 m/sec
:
D
vmax = √3RTM⇒Vmax∞√TM
v2v1 = √M1M2×T2T1√12×12 ⇒v2=v12=3002=150m/sec
:
D
vmax = √3RTM⇒Vmax∞√TM
v2v1 = √M1M2×T2T1√12×12 ⇒v2=v12=3002=150m/sec