12th Grade > Physics
KINETIC THEORY OF GASES MCQs
Total Questions : 24
| Page 1 of 3 pages
Answer: Option D. -> 1−III,2−II,3−I
:
D
Take 2-3 minutes and try to get this on your own. Then move on the next video for the explanation.
:
D
Take 2-3 minutes and try to get this on your own. Then move on the next video for the explanation.
Answer: Option B. -> Open system
:
B
Let's talk about the different kinds of system first.
Open system -matter and energy both can be transferred.
Closed system - Only energy can be transferred.
Isolated - Neither energy nor matter can be transferred.
A mug is a vessel, it allows for the exchange of matter and energy between the system and surroundings. Thus it is an open system.
:
B
Let's talk about the different kinds of system first.
Open system -matter and energy both can be transferred.
Closed system - Only energy can be transferred.
Isolated - Neither energy nor matter can be transferred.
A mug is a vessel, it allows for the exchange of matter and energy between the system and surroundings. Thus it is an open system.
Answer: Option B. -> 636∘C
:
B
Since volume is constant,
Hence P1P2=T1T2⇒13=(273+30)T2
⇒ T2 = 909 K = 636∘C
:
B
Since volume is constant,
Hence P1P2=T1T2⇒13=(273+30)T2
⇒ T2 = 909 K = 636∘C
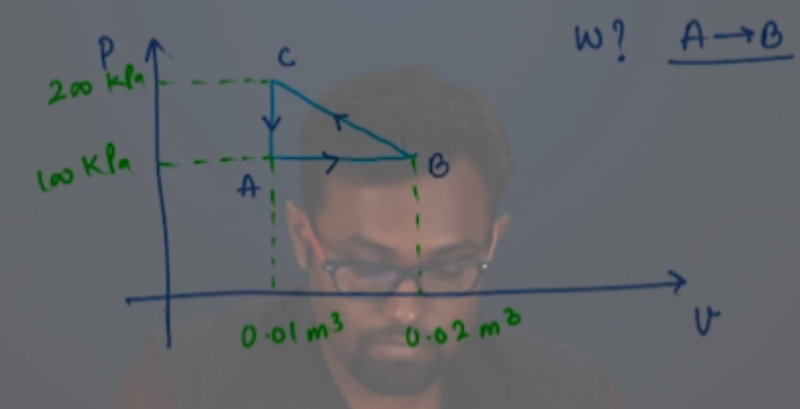
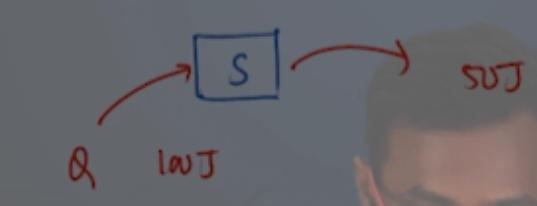
Answer: Option C. -> +50 J
:
C
Take a minute and try to get this on your own. Then move on the next video for the explanation.
:
C
Take a minute and try to get this on your own. Then move on the next video for the explanation.
Answer: Option C. -> T1T2(P1V1+P2V2)P1V1T2+P2V2T1)
:
C
According to the kinetic theory, the average kinetic energy(KE) per molecule of a gas
= 32KT. Let n1 and n2 be the number of moles of air in vessels 1 and 2 respectively.
Before mixing, the total KE of molecules in the two vessels is
E1=32n1kT1+32n2kT2
= 32k(n1T1+n2T2)
After mixing, the total KE of molecules is
E2=32(n1+n2)kT
Where T is the temperature when equilibrium is established. Since there is no loss of energy
(Because the vessels are insulated), E2=E1 or
32(n1+n2)kT=32k(n1T1+n2T2)
or T=n1T1+n2T2n1+n2
Now P1V1=n1RT1 andP2V2=n2RT2 which give
n1=P1V1RT1 and n2=P2V2RT2
Using these in Eq. (1) and simplifying, we get
T=T1T2(P1V1+P2V2)(P1V1T2+P2V2T1)
:
C
According to the kinetic theory, the average kinetic energy(KE) per molecule of a gas
= 32KT. Let n1 and n2 be the number of moles of air in vessels 1 and 2 respectively.
Before mixing, the total KE of molecules in the two vessels is
E1=32n1kT1+32n2kT2
= 32k(n1T1+n2T2)
After mixing, the total KE of molecules is
E2=32(n1+n2)kT
Where T is the temperature when equilibrium is established. Since there is no loss of energy
(Because the vessels are insulated), E2=E1 or
32(n1+n2)kT=32k(n1T1+n2T2)
or T=n1T1+n2T2n1+n2
Now P1V1=n1RT1 andP2V2=n2RT2 which give
n1=P1V1RT1 and n2=P2V2RT2
Using these in Eq. (1) and simplifying, we get
T=T1T2(P1V1+P2V2)(P1V1T2+P2V2T1)
Answer: Option C. -> 2P
:
C
For a gas, PV=nRT. Hence
(P)O2=(1mole)RTV
and (P)He=(1mole)R(2T)V
∴(P)He(P)O2=2 or (P)He=2(P)O2
:
C
For a gas, PV=nRT. Hence
(P)O2=(1mole)RTV
and (P)He=(1mole)R(2T)V
∴(P)He(P)O2=2 or (P)He=2(P)O2
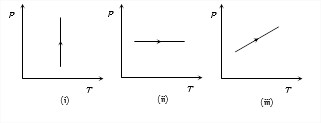
Answer: Option C. -> Density of gas is constant in graph (iii)
:
C
(v) μ = PMRT
Density μ remains constant when PT or volume remains constant.
In graph (i) Pressure is increasing at constant temperature hence volume is decreasing so density is increasing. Graphs (ii) and (iii) volume is increasing hence, density is decreasing. Not that
volume whould had been constant in case the stright line in graph (iii) had passed through origin
:
C
(v) μ = PMRT
Density μ remains constant when PT or volume remains constant.
In graph (i) Pressure is increasing at constant temperature hence volume is decreasing so density is increasing. Graphs (ii) and (iii) volume is increasing hence, density is decreasing. Not that
volume whould had been constant in case the stright line in graph (iii) had passed through origin
Answer: Option D. -> quasi-static process
:
D
In thermodynamics, a quasi-static process is a thermodynamic process that happens slowly enough for the system to remain in internal equilibrium. Only in such processes can each moment be represented on the P-Vdiagram.
:
D
In thermodynamics, a quasi-static process is a thermodynamic process that happens slowly enough for the system to remain in internal equilibrium. Only in such processes can each moment be represented on the P-Vdiagram.
Question 9. The Bat Life: Batman is undercover, and to prevent anyone from recognizing his voice (or Bruce Wayne's) he inhales a very dilute Helium gas (search YouTube for "voice after helium” to see the effects). The helium is stored ina cylindrical chamber as shown.
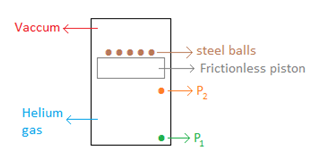
During a fight one of the steel balls gets dislodged. What will happen to the piston?
During a fight one of the steel balls gets dislodged. What will happen to the piston?
Answer: Option B. -> It will move up
:
B
Initial free body diagram -
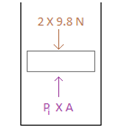 .
.
When the system was in equilibrium, the force applied by the steel balls on the piston was offset by the force applied by the gas on the piston.
Just after removing the ball -
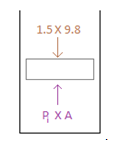 .
.
The instant the ball is dislodged the downward force acting on the piston reduces from 19.6 N (i.e.,2×9.8N)to14.7N(1.5×9.8N). However the pressure of the helium gas has not changed. Hence there is an unbalanced force in the upward direction. This upward force will push the piston up. After some time, the weight of the balls would equal the force exerted by the helium gas and the system would eventually come to rest.
:
B
Initial free body diagram -
When the system was in equilibrium, the force applied by the steel balls on the piston was offset by the force applied by the gas on the piston.
Just after removing the ball -
The instant the ball is dislodged the downward force acting on the piston reduces from 19.6 N (i.e.,2×9.8N)to14.7N(1.5×9.8N). However the pressure of the helium gas has not changed. Hence there is an unbalanced force in the upward direction. This upward force will push the piston up. After some time, the weight of the balls would equal the force exerted by the helium gas and the system would eventually come to rest.
Answer: Option B. -> It will move up
:
C
Let us analyse each process separately. BC is an isothermal process, which implies that the P-Cgraph would be represented by a rectangular hyperbola. (Since PV= constant).
This rectangular hyperbola starts at a lower volume V1 and goes to a higher volume as represented in the figure below -
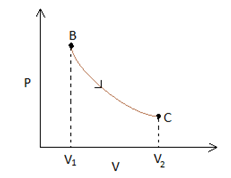
Process AB is an isochoric process, which in a P-Vgraph would be a vertical line. Also we notice that the temperature drops in this process, whichimplies that the pressure will also drop (since PV=nRT, when Vis constant P∝T). Therefore point Acan be anywhere on the line represented in blue in the figure below.
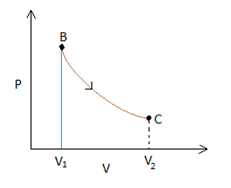
Process C - A
This process is an isobaric process since it represents a curve where V∝T (this is possible only when pressure is a constant, remember Charles' law?). So the process can be represented by a horizontal line in a P-Vgraph. The point where the line joining process A-Band C-Ameet is the point A.
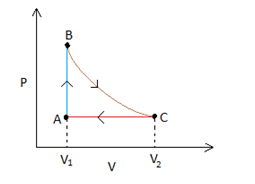
:
C
Let us analyse each process separately. BC is an isothermal process, which implies that the P-Cgraph would be represented by a rectangular hyperbola. (Since PV= constant).
This rectangular hyperbola starts at a lower volume V1 and goes to a higher volume as represented in the figure below -
Process AB is an isochoric process, which in a P-Vgraph would be a vertical line. Also we notice that the temperature drops in this process, whichimplies that the pressure will also drop (since PV=nRT, when Vis constant P∝T). Therefore point Acan be anywhere on the line represented in blue in the figure below.
Process C - A
This process is an isobaric process since it represents a curve where V∝T (this is possible only when pressure is a constant, remember Charles' law?). So the process can be represented by a horizontal line in a P-Vgraph. The point where the line joining process A-Band C-Ameet is the point A.