Question
Answer: Option C
:
C
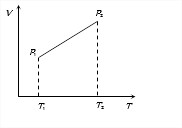
In case of given graph, V and T are related as V = aT +b, where a and b are constants.
From ideal gas equation, PV = μRT
We find P = μRTaT+b = μa+bT
Since T2>T1, therefore P2>P1.
Was this answer helpful ?
:
C
In case of given graph, V and T are related as V = aT +b, where a and b are constants.
From ideal gas equation, PV = μRT
We find P = μRTaT+b = μa+bT
Since T2>T1, therefore P2>P1.
Was this answer helpful ?


















Submit Solution