Question
If we allow current to pass through the given set up for some time, which of the following can be observed? 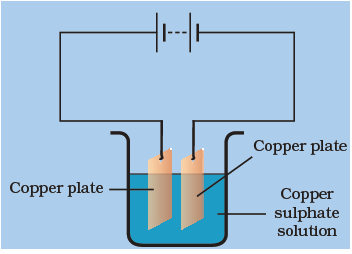

Answer: Option C
:
C
When electric current is passed through the copper sulphate solution, copper sulphate dissociates into Cu2+ and SO2−4 ions. The Cu2+ get drawn to the electrode connected to the negative terminal of the battery and gets deposited on it.
Was this answer helpful ?
:
C
When electric current is passed through the copper sulphate solution, copper sulphate dissociates into Cu2+ and SO2−4 ions. The Cu2+ get drawn to the electrode connected to the negative terminal of the battery and gets deposited on it.
Was this answer helpful ?


















Submit Solution