MCQs
Laws Of Thermodynamics
Total Questions : 1462
| Page 3 of 147 pages
Answer: Option C. -> TH−TLTH
:
C
The work done by the gas over the entire cycle divided by the heat consumed from the hot reservoir is the efficiency of the Carnot engine.
Let us consider each isothermal process and find the work done in each of them.
Process a - b (isothermal)
Work done in an isothermal process is given by the formula
W=nRTℓn(v2v1)
In this case
W1=nRTℓn(vnvb). . . . (1)
Similarly the work done in process c - d is
W3=nRTℓn(vcvd). . . . (2)
Since both of these process are isothermal
QL=W3...(4)andQH=W1...(5)
The Carnot engine is represented below
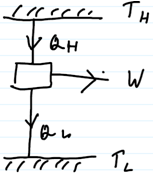
Applying law of conservation of energy we get
QH−QL=W
Dividing throughout by QH we get
1−QLQH=WQH=η. . . . .(6)
Substituting for QH and QL in(6)
We get
1−nRTLln(vcvd)nRTHln(vbva)=η
⇒η=1−TLTHln(vcvd)(vbva)
In process b - c since it is adiabatic the state variables T and V are connected using the relation
T(1γ−1)b×Vb=T(1γ−1)c×Vc ......(8)
and similarly
T(1γ−1)d×Vd=T(1γ−1)a×Va .......(9)
From (8) and we get
vcvd=vbva
⇒ln(vcvd)ln(vbva) .......(10)
Substituting (10) in (9) we get
eta=1−TL
η=1−TLTH
:
C
The work done by the gas over the entire cycle divided by the heat consumed from the hot reservoir is the efficiency of the Carnot engine.
Let us consider each isothermal process and find the work done in each of them.
Process a - b (isothermal)
Work done in an isothermal process is given by the formula
W=nRTℓn(v2v1)
In this case
W1=nRTℓn(vnvb). . . . (1)
Similarly the work done in process c - d is
W3=nRTℓn(vcvd). . . . (2)
Since both of these process are isothermal
QL=W3...(4)andQH=W1...(5)
The Carnot engine is represented below
Applying law of conservation of energy we get
QH−QL=W
Dividing throughout by QH we get
1−QLQH=WQH=η. . . . .(6)
Substituting for QH and QL in(6)
We get
1−nRTLln(vcvd)nRTHln(vbva)=η
⇒η=1−TLTHln(vcvd)(vbva)
In process b - c since it is adiabatic the state variables T and V are connected using the relation
T(1γ−1)b×Vb=T(1γ−1)c×Vc ......(8)
and similarly
T(1γ−1)d×Vd=T(1γ−1)a×Va .......(9)
From (8) and we get
vcvd=vbva
⇒ln(vcvd)ln(vbva) .......(10)
Substituting (10) in (9) we get
eta=1−TL
η=1−TLTH
Answer: Option D. -> 50000 J
:
D
QHOT=W+QCOLD=41000J+9000J=50000J
:
D
QHOT=W+QCOLD=41000J+9000J=50000J
Answer: Option B. -> 1117
:
B
The efficiency of the engine can be calculated by the formula in the hint
η=1−300850
=1−617=1117
:
B
The efficiency of the engine can be calculated by the formula in the hint
η=1−300850
=1−617=1117
Answer: Option A. -> heat was input
:
A
Along AB heat was input. Along the line AB, the quantity pV increased, so NkT increased so ΔU=NkΔT was positive. ΔU=Q+W, and W = 0 since pΔV=0. So ΔU=Q is positive.
:
A
Along AB heat was input. Along the line AB, the quantity pV increased, so NkT increased so ΔU=NkΔT was positive. ΔU=Q+W, and W = 0 since pΔV=0. So ΔU=Q is positive.
Question 25. In the P-Vdiagram show, moles of a gas are taken through a process from state i to f, at constant pressure. Part of the heat must have gone into doing the work PΔV, and the rest in increasing the internal energy. If the molar specific heat at constant volume for the gas is CV, and the initial total internal energy is Ui, what is the final internal energy Uf?
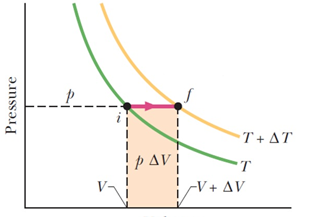
Answer: Option D. -> Uf = Ui + CvΔT
:
D
: In the diagram, the two lines represent isotherms for temperatures T and (T+ΔT) - any point on either line will be at the same temperature. If a process takes the state of a gas from a point on one isotherm to a point on another isotherm, the change in internal energy will be related to the temperature difference only, and nothing else, given by the relation -
ΔEint=nCvΔT
Therefore in our case,
Uf=Ui+ΔEint
⇒Uf=Ui+nCvΔT.
:
D
: In the diagram, the two lines represent isotherms for temperatures T and (T+ΔT) - any point on either line will be at the same temperature. If a process takes the state of a gas from a point on one isotherm to a point on another isotherm, the change in internal energy will be related to the temperature difference only, and nothing else, given by the relation -
ΔEint=nCvΔT
Therefore in our case,
Uf=Ui+ΔEint
⇒Uf=Ui+nCvΔT.
Answer: Option C. -> 600 J
:
C
The refrigerator must reject an amount of heat to the room equal to the sum of the work done by the refrigerator and the heat removed from its contents or 400 J + 200 J = 600 J
:
C
The refrigerator must reject an amount of heat to the room equal to the sum of the work done by the refrigerator and the heat removed from its contents or 400 J + 200 J = 600 J
Answer: Option B. -> greater than or equal to zero
:
B
Greater than or equal to zero. By the 2nd Law, the entropy of the universe (engine + hot bath + cold bath) can never decrease. The entropy of the engine remains constant (see previous question) so the entropy of the reservoirs must increase. In the case of an ideal Carnot cycle, the process is reversible and the net entropy change is zero.
:
B
Greater than or equal to zero. By the 2nd Law, the entropy of the universe (engine + hot bath + cold bath) can never decrease. The entropy of the engine remains constant (see previous question) so the entropy of the reservoirs must increase. In the case of an ideal Carnot cycle, the process is reversible and the net entropy change is zero.
Question 28. We know that, for an ideal monoatomic gas, the total internal energy is a result of the translational kinetic energy only. For the oxygen molecule O2 (assume it doesn't oscillate along the bond axis), which rotates as well as translates, choose the correct statement(s) from the following
Answer: Option C. -> Translational kinetic energy > rotational kinetic energy
:
C
The equipartition theorem postulates - each degree of freedom contributes an energy equal to (12)kT to the total internal energy of a molecule, depending on the temperature T. Let's count the number of degrees of freedom for an O2 molecule.
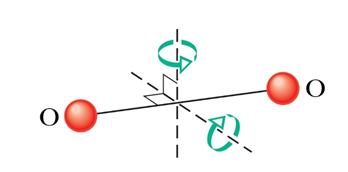
(1) Possible directions of translational motion are the three axes, x, y and z. Thus, number of translational degrees of motion = 3.
(2) Given the negligible size of the atoms, rotation about the bond-axis will not be significant. It can only rotate about the two axes shown (perpendicular to the bond-axis). Thus number of rotational degrees of motion = 2.
Therefore, according the equipartition theorem,
(i) Translational Kinetic energy, KTr=32kT,
(ii) Rotational Kinetic energy, KRot=kT.
Clearly, KTr > KRot.
:
C
The equipartition theorem postulates - each degree of freedom contributes an energy equal to (12)kT to the total internal energy of a molecule, depending on the temperature T. Let's count the number of degrees of freedom for an O2 molecule.
(1) Possible directions of translational motion are the three axes, x, y and z. Thus, number of translational degrees of motion = 3.
(2) Given the negligible size of the atoms, rotation about the bond-axis will not be significant. It can only rotate about the two axes shown (perpendicular to the bond-axis). Thus number of rotational degrees of motion = 2.
Therefore, according the equipartition theorem,
(i) Translational Kinetic energy, KTr=32kT,
(ii) Rotational Kinetic energy, KRot=kT.
Clearly, KTr > KRot.
Answer: Option D. -> 7/3
Answer: (d).7/3
Answer: (d).7/3
Answer: Option D. -> all of these
Answer: (d).all of these
Answer: (d).all of these
















