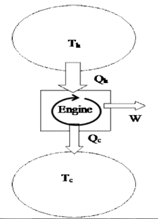
Question
In one cycle of the engine, the net change in the entropy of both reservoirs (hot and cold together) is:
Answer: Option B
:
B
Greater than or equal to zero. By the 2nd Law, the entropy of the universe (engine + hot bath + cold bath) can never decrease. The entropy of the engine remains constant (see previous question) so the entropy of the reservoirs must increase. In the case of an ideal Carnot cycle, the process is reversible and the net entropy change is zero.
Was this answer helpful ?
:
B
Greater than or equal to zero. By the 2nd Law, the entropy of the universe (engine + hot bath + cold bath) can never decrease. The entropy of the engine remains constant (see previous question) so the entropy of the reservoirs must increase. In the case of an ideal Carnot cycle, the process is reversible and the net entropy change is zero.
Was this answer helpful ?
More Questions on This Topic :
Question 4. The value of 1 mm of Hg is equal to....
Question 7. Which of the following is correct?....


















Submit Solution