Question
We know that, for an ideal monoatomic gas, the total internal energy is a result of the translational kinetic energy only. For the oxygen molecule O2 (assume it doesn't oscillate along the bond axis), which rotates as well as translates, choose the correct statement(s) from the following
Answer: Option C
:
C
The equipartition theorem postulates - each degree of freedom contributes an energy equal to (12)kT to the total internal energy of a molecule, depending on the temperature T. Let's count the number of degrees of freedom for an O2 molecule.
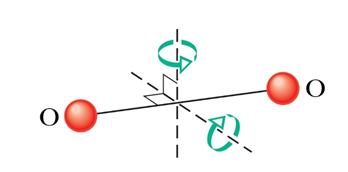
(1) Possible directions of translational motion are the three axes, x, y and z. Thus, number of translational degrees of motion = 3.
(2) Given the negligible size of the atoms, rotation about the bond-axis will not be significant. It can only rotate about the two axes shown (perpendicular to the bond-axis). Thus number of rotational degrees of motion = 2.
Therefore, according the equipartition theorem,
(i) Translational Kinetic energy, KTr=32kT,
(ii) Rotational Kinetic energy, KRot=kT.
Clearly, KTr > KRot.
Was this answer helpful ?
:
C
The equipartition theorem postulates - each degree of freedom contributes an energy equal to (12)kT to the total internal energy of a molecule, depending on the temperature T. Let's count the number of degrees of freedom for an O2 molecule.
(1) Possible directions of translational motion are the three axes, x, y and z. Thus, number of translational degrees of motion = 3.
(2) Given the negligible size of the atoms, rotation about the bond-axis will not be significant. It can only rotate about the two axes shown (perpendicular to the bond-axis). Thus number of rotational degrees of motion = 2.
Therefore, according the equipartition theorem,
(i) Translational Kinetic energy, KTr=32kT,
(ii) Rotational Kinetic energy, KRot=kT.
Clearly, KTr > KRot.
Was this answer helpful ?
More Questions on This Topic :
Question 3. The value of 1 mm of Hg is equal to....
Question 6. Which of the following is correct?....


















Submit Solution