12th Grade > Chemistry
P BLOCK ELEMENTS MCQs
Total Questions : 30
| Page 3 of 3 pages
Answer: Option B. -> NH4Cl+Na2B4O7RedHot−−−−→
:
B
2NH4Cl+Na2B4O7Δ−→2BN+B2O3+2NaCl+4H2O
:
B
2NH4Cl+Na2B4O7Δ−→2BN+B2O3+2NaCl+4H2O
Answer: Option D. -> CuSO4.Al2(SO4)3.24H2O
:
D
Alums contain monovalent M+ and trivalent M+3 ions CuSO4.Al2(SO4)3.24H2O has Cu+2 and Al+3 ions
:
D
Alums contain monovalent M+ and trivalent M+3 ions CuSO4.Al2(SO4)3.24H2O has Cu+2 and Al+3 ions
Answer: Option A. -> It is a tribasic acid
:
A
H3BO3 is weak monobasic acid
:
A
H3BO3 is weak monobasic acid
Answer: Option B. -> SiO2,CO
:
B
SiO2+2C→Si+2CO.
:
B
SiO2+2C→Si+2CO.
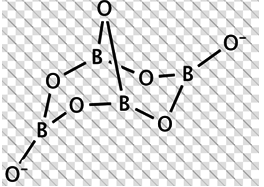
Question 27. Borax is actually made of two tetrahedra and two triangular units joined together and should be written as: Na2[B4O5(OH)4]·8H2O
The correct statement/s about borax is/are:
a. Each boron atom has four B-O bonds
b. Each boron atom has three B-O bonds
c. Two boron atoms have four B-O bonds while other two have three B-O bonds
d. Each boron atom has one -OH groups
The correct statement/s about borax is/are:
a. Each boron atom has four B-O bonds
b. Each boron atom has three B-O bonds
c. Two boron atoms have four B-O bonds while other two have three B-O bonds
d. Each boron atom has one -OH groups
Answer: Option A. -> X = Metaboric acid and Y = Tetraboric acid
:
A
X=Metaboricacid=HBO2Y = Tetraboricacid=H2B4O7
:
A
X=Metaboricacid=HBO2Y = Tetraboricacid=H2B4O7
Answer: Option B. -> BBr3>BCl3>BF3
:
B
Back bonding tendency decreases from F to I
:
B
Back bonding tendency decreases from F to I
Answer: Option C. -> It forms R2O3
:
C
13th group elements have 3 valency electrons hence they can show oxidation state +3 and +1
:
C
13th group elements have 3 valency electrons hence they can show oxidation state +3 and +1