Question
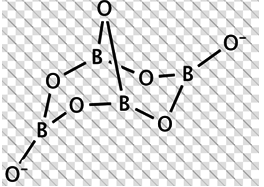
Borax is actually made of two tetrahedra and two triangular units joined together and should be written as: Na2[B4O5(OH)4]·8H2O
The correct statement/s about borax is/are:
a. Each boron atom has four B-O bonds
b. Each boron atom has three B-O bonds
c. Two boron atoms have four B-O bonds while other two have three B-O bonds
d. Each boron atom has one -OH groups
The correct statement/s about borax is/are:
a. Each boron atom has four B-O bonds
b. Each boron atom has three B-O bonds
c. Two boron atoms have four B-O bonds while other two have three B-O bonds
d. Each boron atom has one -OH groups


















Submit Solution