Question
Which of the following is the correct order of the atomic size of C, N, P and S?
Answer: Option A
:
A
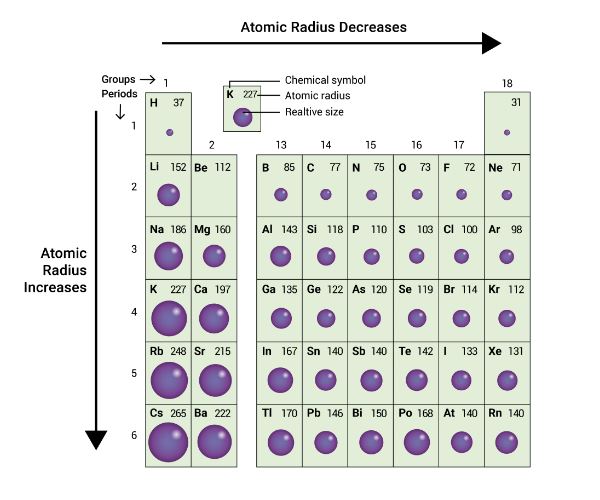
In a group, the size of an atom increases as one proceeds from top to bottom.
This is due to the successive addition of shells.
In a period, the size of an atom decreases from left to right.
This is because the effective nuclear charge increases from left to right in the same period, thereby bringing the outermost shell closer to the nucleus.
So, the correct order of atomic size are as follows:
N < C < S < P
Was this answer helpful ?
:
A
In a group, the size of an atom increases as one proceeds from top to bottom.
This is due to the successive addition of shells.
In a period, the size of an atom decreases from left to right.
This is because the effective nuclear charge increases from left to right in the same period, thereby bringing the outermost shell closer to the nucleus.
So, the correct order of atomic size are as follows:
N < C < S < P
Was this answer helpful ?


















Submit Solution