Question
Answer: Option B
:
B
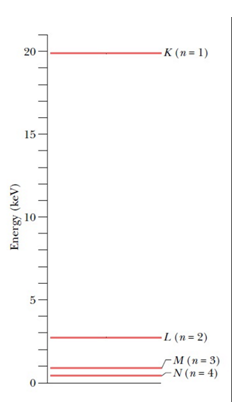
The energies marked in the diagram are the energies of the atom when a vacancy of electron is created in particular shells.
For example, the energy marked as EKis the energy of atom when one electron from the K shell is removed. And so on.
It is interesting to note that the energy of the atom with one vacancy in K shell is higher; and as we move towards the higher shells, the energies of the atom with vacancies in those shells decrease. Also, the energy is zero when the atom is in its ground state.
This can be understood simply, as the K shell electrons are bound to the nucleus most strongly; this force decreases as we move to higher shells. Hence, to remove an electron from the lowermost shell (K) maximum amount of energy is required to be supplied, this amount will decrease as we move towards higher shells; for the same reason.
In the ground state (no electrons removed) the atom’s energy will be the least, which is taken to be zero.
Was this answer helpful ?
:
B

The energies marked in the diagram are the energies of the atom when a vacancy of electron is created in particular shells.
For example, the energy marked as EKis the energy of atom when one electron from the K shell is removed. And so on.
It is interesting to note that the energy of the atom with one vacancy in K shell is higher; and as we move towards the higher shells, the energies of the atom with vacancies in those shells decrease. Also, the energy is zero when the atom is in its ground state.
This can be understood simply, as the K shell electrons are bound to the nucleus most strongly; this force decreases as we move to higher shells. Hence, to remove an electron from the lowermost shell (K) maximum amount of energy is required to be supplied, this amount will decrease as we move towards higher shells; for the same reason.
In the ground state (no electrons removed) the atom’s energy will be the least, which is taken to be zero.
Was this answer helpful ?
More Questions on This Topic :
Question 4. X−KαY−KβZ−Kγ....


















Submit Solution