Question
Answer: Option D
:
D
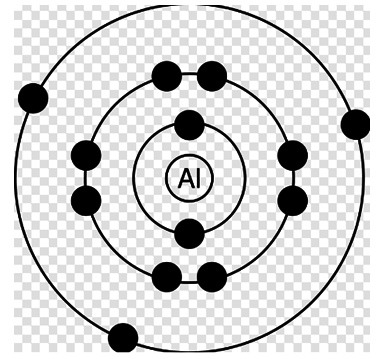
It’s a very simple question. We have to compare the energy in all the four diagrams. Basically these diagrams represent an atom with different electronic configuration with, infact, an electron missing from a specific shell in each case. Proceeding organically, first of all let's say the energy in the first case (with each electron at its place) is x.
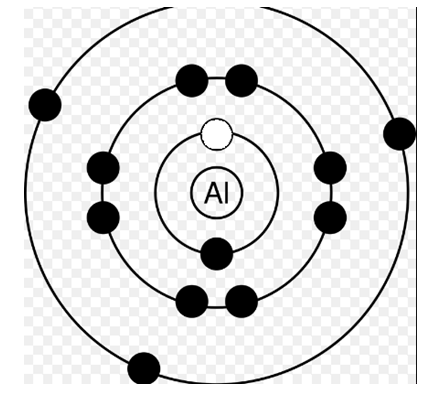
Now, in the second diagram the electron is removed from the K-shell. We need to supply energy to the atom to do this right? So let's say we supplied an energy ΔK.
So, the atom's energy now becomes x+ΔK.
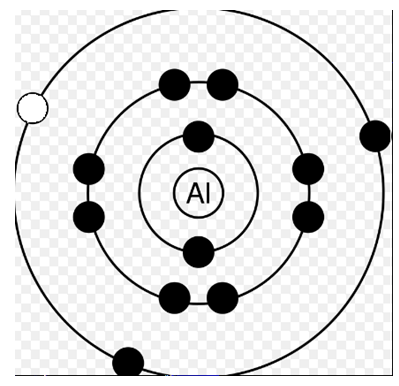
Now in the third diagram the electron is removed from M shell. We need to supply energy even for that. Let's say that energy is ΔM.
So, atom’s energy becomes x+ΔM.
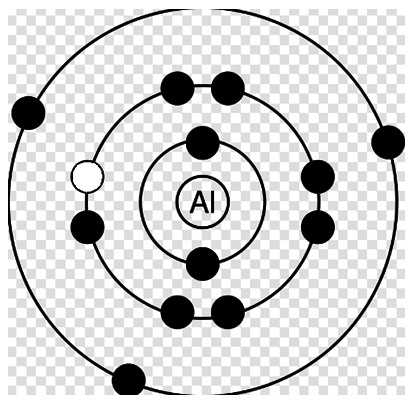
In the fourth diagram, similarly after removing electron from L-shell the atomic energy becomes x+ΔL.
Now, we understand that it is tougher to remove the electron which is closer to the nucleus as it is more strongly bounded with the nucleus. To remove the electron from K-shell we need to supply the highest amount of energy, and to remove an electron from the M-shell we need to supply the least amount of energy. Mathematically,ΔK >ΔL >ΔM
Hence the energy of the atom will be in the order: II > IV > III > I.
Was this answer helpful ?
:
D
It’s a very simple question. We have to compare the energy in all the four diagrams. Basically these diagrams represent an atom with different electronic configuration with, infact, an electron missing from a specific shell in each case. Proceeding organically, first of all let's say the energy in the first case (with each electron at its place) is x.
Now, in the second diagram the electron is removed from the K-shell. We need to supply energy to the atom to do this right? So let's say we supplied an energy ΔK.
So, the atom's energy now becomes x+ΔK.
Now in the third diagram the electron is removed from M shell. We need to supply energy even for that. Let's say that energy is ΔM.
So, atom’s energy becomes x+ΔM.
In the fourth diagram, similarly after removing electron from L-shell the atomic energy becomes x+ΔL.
Now, we understand that it is tougher to remove the electron which is closer to the nucleus as it is more strongly bounded with the nucleus. To remove the electron from K-shell we need to supply the highest amount of energy, and to remove an electron from the M-shell we need to supply the least amount of energy. Mathematically,ΔK >ΔL >ΔM
Hence the energy of the atom will be in the order: II > IV > III > I.
Was this answer helpful ?


















Submit Solution