9th Grade > Chemistry
STRUCTURE OF AN ATOM MCQs
:
A and C
∙ Isotopes have the same atomic number (same number of protons) and different mass number.
∙ Since, 11H, 21H, 31H have the same atomic number, they are isotopes of hydrogen.
∙ Similarly, 126C, 136C, 146C have same atomic number, hence they are isotopes of carbon.
∙ Whereas, 4020Ca, 4018Ar and 5826Fe, 5827Ni have different atomic numbers and hence, are not isotopes. They are isobars as they have same mass number.
:
C
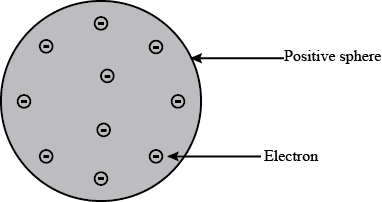
Thomson proposed that :
1) An atom consists of a positively charged sphere and the electrons are embedded in it.
2) The negative and positive charges are equal in magnitude.
From above two we can say that the atom as a whole is electrically neutral.
:
A
Neutrons are fundamental sub-atomic particles present in the nucleus of an atom. Hydrogen is the only element which is devoid of neutrons.
:
A
According to Thomson's atomic model, the positive charge is uniformly distributed in the sphere and electrons are embedded in it just like a plum pudding.This implies that when alpha particles are bombarded on the atoms, they should get deflected uniformly.
However, in the Rutherford alpha particle scattering experiment, it was found that rays were deflected in various angles.
:
D
Following are the properties of anode rays:
1) Anode rays deflect towards negatively charged plate.
2) Anode rays travel along a straight path in absence of electric and magnetic field.
3) Anode rays are positively charged radiations due to positive charged sub-atomic particles.
:
A
Atoms have electrons, protons, and neutrons as the fundamental particles.
Subatomic particleMass (kg)Proton1.67262178 × 10−27Neutron 1.6749 × 10−27Electron 9.10938291 × 10−31
Mass of protonMass of electron= 1.67262178×10−279.10938291×10−31 = 1840
Thus, mass of electron is 11840, i.e., 0.0005 times that of proton.
Hence, the mass of an electron is negligible when compared to the mass of proton.
:
B and D
According to Rutherford, the atoms are made of two parts: the nucleus and the extra-nuclear part. His experiments proved that the atom is largely empty and has a heavy positively-charged body at the centre called the nucleus. The central nucleus is positively-charged and the negatively-charged electrons revolve around the nucleus.
:
A
J.J Thomson was the first scientist who measured charge to mass ratio(e/m) of an electron. When a narrow beam of charged particles are projected at constant speed (v) across a magnetic field in a direction perpendicular to the field, the beam of particles experiences a force, which makes them move in a circular path.
:
D
An electron is a negatively charged subatomic particle.
It has a charge of −1.602×10−19 coulombs.