9th Grade > Chemistry
STRUCTURE OF AN ATOM MCQs
Total Questions : 53
| Page 6 of 6 pages
Answer: Option C. ->
Malleability
:
C
The ability of the metal to be beaten into thin sheets is called malleability. Rutherford needed a very thin sheet of metal for the alpha-ray scattering experiment. Hence, gold, the most malleable metal was used.
:
C
The ability of the metal to be beaten into thin sheets is called malleability. Rutherford needed a very thin sheet of metal for the alpha-ray scattering experiment. Hence, gold, the most malleable metal was used.
Answer: Option B. ->
The orbit's or shell's serial number
:
B
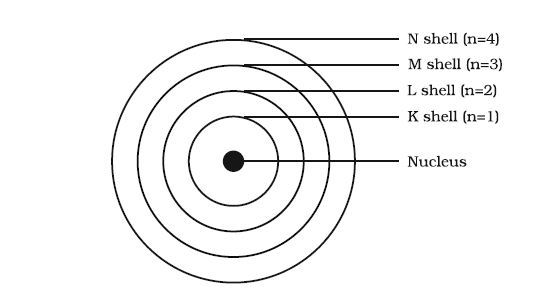
According to Niels Bohr, the shells or orbits in which electrons are present in an atom are represented by K (n=1), L (n=2), M (n=3), N (n=4). The term, 'n' represent the orbit's number. For instance, n=1 represents the first orbit; n=2 represents the second orbit.
:
B
According to Niels Bohr, the shells or orbits in which electrons are present in an atom are represented by K (n=1), L (n=2), M (n=3), N (n=4). The term, 'n' represent the orbit's number. For instance, n=1 represents the first orbit; n=2 represents the second orbit.