9th Grade > Chemistry
STRUCTURE OF AN ATOM MCQs
Total Questions : 53
| Page 1 of 6 pages
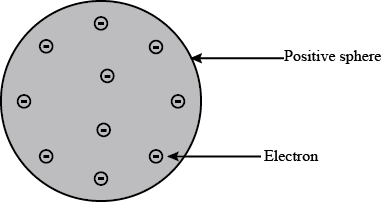
Answer: Option C. -> An atom consists of a positively charged sphere and electrons are embedded in it, the positive and negative charges are equal in magnitude.
:
C
Thomson proposed that :
1) An atom consists of a positively charged sphere and the electrons are embedded in it.
2) The negative and positive charges are equal in magnitude.
From above two we can say that the atom as a whole is electrically neutral.
:
C
Thomson proposed that :
1) An atom consists of a positively charged sphere and the electrons are embedded in it.
2) The negative and positive charges are equal in magnitude.
From above two we can say that the atom as a whole is electrically neutral.
Answer: Option B. -> False
:
B
The radioactivity has medical and industrial applications as well. An isotope of uranium is used as a fuel in nuclear reactors. An isotope of cobalt is used in the treatment of cancer. Hence the given statement is false.
:
B
The radioactivity has medical and industrial applications as well. An isotope of uranium is used as a fuel in nuclear reactors. An isotope of cobalt is used in the treatment of cancer. Hence the given statement is false.
Answer: Option B. -> The orbit's or shell's serial number
:
B
According to Niels Bohr, the shells or orbits in which electrons are present in an atom are represented by K (n=1), L (n=2), M (n=3), N (n=4). The term, 'n' represent the orbit's number. For instance, n=1 represents thefirst orbit; n=2 represents thesecond orbit.
:
B
According to Niels Bohr, the shells or orbits in which electrons are present in an atom are represented by K (n=1), L (n=2), M (n=3), N (n=4). The term, 'n' represent the orbit's number. For instance, n=1 represents thefirst orbit; n=2 represents thesecond orbit.
Answer: Option A. -> Electrons move to higher energy orbits by absorbing energy
:
A
Bohr's Model of the atom included the idea(s) that:
The electron can have only certain energies, including a lowest-level ground state, electronsemit energy as light when they move to the lower energy orbits and electrons absorb energy by moving to the higher energy orbits.
:
A
Bohr's Model of the atom included the idea(s) that:
The electron can have only certain energies, including a lowest-level ground state, electronsemit energy as light when they move to the lower energy orbits and electrons absorb energy by moving to the higher energy orbits.
Answer: Option D. -> 2n2
:
D
The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is always natural number.
n represents the principal quantum number. It represents the size and the energy of the shell.
Hence, the maximum number of electrons in different shells are as follows:
1. First orbit or K-shell (n=1) =
2x12 = 2
2. Second orbit or L-shell (n=2) =
2x22 = 8
and so on.
:
D
The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is always natural number.
n represents the principal quantum number. It represents the size and the energy of the shell.
Hence, the maximum number of electrons in different shells are as follows:
1. First orbit or K-shell (n=1) =
2x12 = 2
2. Second orbit or L-shell (n=2) =
2x22 = 8
and so on.
Answer: Option A. -> Neutrons
:
A
Neutrons are fundamental sub-atomic particles present in the nucleus of an atom. Hydrogen is the only element which is devoid of neutrons.
:
A
Neutrons are fundamental sub-atomic particles present in the nucleus of an atom. Hydrogen is the only element which is devoid of neutrons.
Answer: Option C. -> Canal rays
:
C
Eugen Goldstein in 1886 discovered the presence of positively charged rays in a gas dischargetube. He called these rays as canal rays.This experiment led to the discovery of protons.
:
C
Eugen Goldstein in 1886 discovered the presence of positively charged rays in a gas dischargetube. He called these rays as canal rays.This experiment led to the discovery of protons.
Answer: Option B. -> Positively charged particles are concentrated at the centre of the atom
:
B
When alpha particles are sent through a thin metal foil only one out of ten thousand rebounded. Rutherford concluded that positively charged particles are concentrated at the centre of the atom.At the time of the experiment, the atom was thought to be analogous to plum pudding(as proposed by J.J. Thomson), with the negative charges (the plums) found throughout a positive sphere (the pudding). If the plum pudding modelwere correct, the positive "pudding", being more spread out than in the current model of a concentrated nucleus, would not be able to exert such large Coulombic forces, and the alpha particles should only be deflected by small angles as they pass through.
:
B
When alpha particles are sent through a thin metal foil only one out of ten thousand rebounded. Rutherford concluded that positively charged particles are concentrated at the centre of the atom.At the time of the experiment, the atom was thought to be analogous to plum pudding(as proposed by J.J. Thomson), with the negative charges (the plums) found throughout a positive sphere (the pudding). If the plum pudding modelwere correct, the positive "pudding", being more spread out than in the current model of a concentrated nucleus, would not be able to exert such large Coulombic forces, and the alpha particles should only be deflected by small angles as they pass through.
Answer: Option A. -> Gold
:
A
In the alpha-ray experiment, Rutherford preferred gold over other metals because it is the most malleable metal. The gold sheet used by Rutherford was about 1000 atoms thick.
:
A
In the alpha-ray experiment, Rutherford preferred gold over other metals because it is the most malleable metal. The gold sheet used by Rutherford was about 1000 atoms thick.