10th Grade > Chemistry
METALS AND NON-METALS MCQs
:
A
Fe (Iron) is the main constituent of haemoglobin. It helps in carrying oxygen to the cells.
:
D
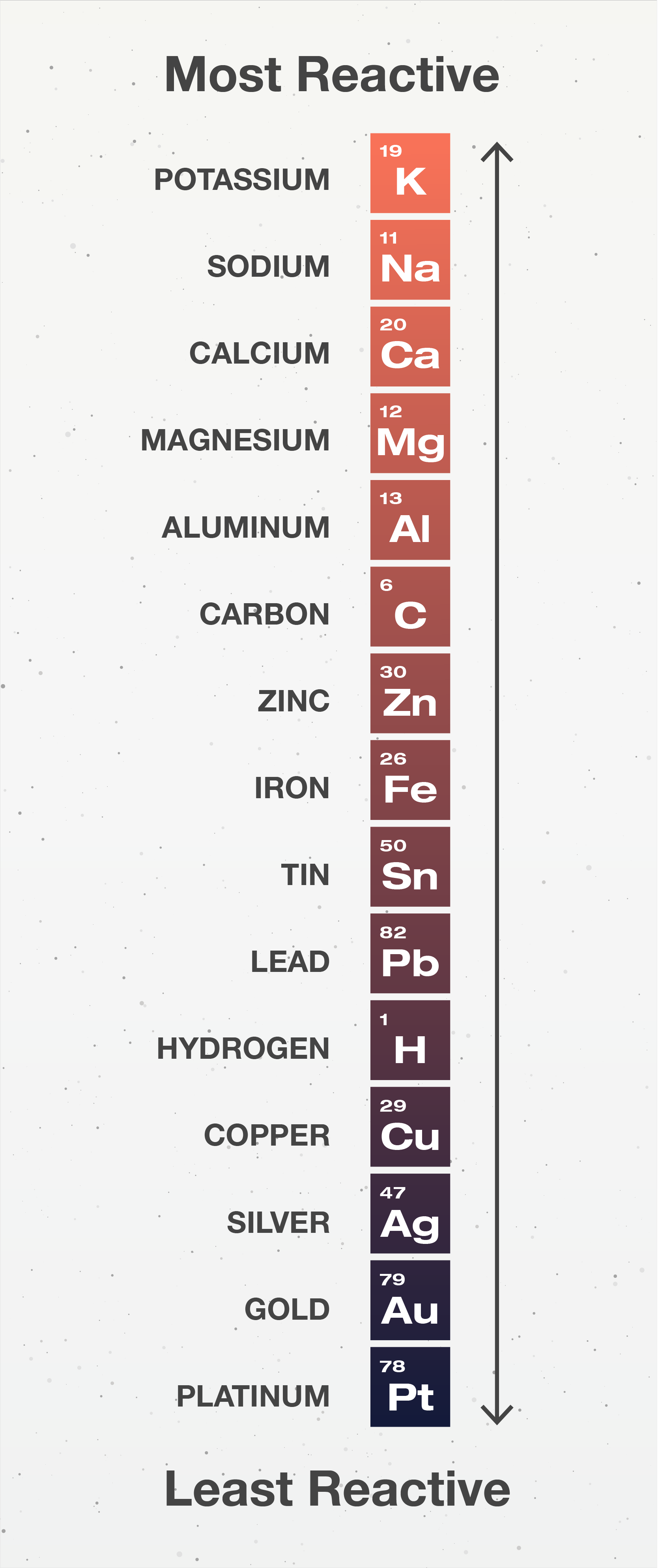
Rusting of iron can be prevented by painting, oiling, greasing, galvanizing, chrome plating, anodizing or making alloys.
Through galvanisation, painting and chrome plating, the surface of the metal is no longer exposed to the atmosphere, and thus, corrosion is prevented.
:
B
Copper Glance ( Cu2S) is a sulphide ore of copper. Rest are oxide ores of copper.
:
B
- Magnetic separation method is used for concentrating ores that contain impurites that have magnetic properties.
- Froath floatation process is used to concentrate sulphide ores.
- Leaching is a process used to concentrate ores that can be dissolved in a chemical solvent.
- Roasting is the process of converting a sulphide ore to an oxide ore by heating it in the presence of oxygen at higher temperatures. This is generally done after concentation of the ore.
:
D
Ionic compounds are made up of positive and negative ions which have strong electrostatic force of attraction among them. So, a lot of heat energy is required to break this force of attraction due to which ionic compounds have high melting point.
:
B
Valency of an atom is the number of electrons it is willing to give up or accept in order to achieve electronic configuration of nearest stable noble gas.
Here, if lithium gives up one electron, it achieves the configuration of helium which is stable. So the valency of lithium is 1.
Similarly, chlorine accepts 1 electron to achieve the configuration of argon. So the valency of chlorine is 1.
:
A
Magnalium is an alloy of aluminium with magnesium and small amounts of nickel and tin.
:
A
Mercury is the only metal that exists as a liquid at room temperature and pressure.
:
C
Malleability refers to the ability of a metal to get beaten or flattened into thin sheets. Gold is the most malleable metal of all. Hence, extremely thin sheets of it can be obtained.
This makes it useful in many applications like shielding from harmful radiation, in jewellery, etc.