9th Grade > Chemistry
MATTER IN OUR SURROUNDINGS MCQs
:
B
Compression means reducing the volume. When we compress something, we are decreasing the volume occupied by it.
When we try to compress a solid, like an iron slab, we would end up either changing the shape or breaking it but we won't really change the volume. This is because the molecules of a solid are very tightly packed. There isn't much space for them to be compressed.
Similarly, if we try to compress a liquid like water it's very difficult to make water occupy a lesser volume. I can't make a litre of water occupy a volume of 500 ml. Of course there is more space between the molecules of a liquid, yet it's still difficult to compress it.
But gases are interesting. Gases have large spaces between the molecules. We can apply pressure and make a gas occupy a lesser space (volume). This is why we say gases are compressible.
:
C
Sublimation is the process by which a substance converts directly from its solid to gaseous state. Examples of sublimes are dry ice (solid CO2), camphor, naphthalene, etc.
:
D
Fluids are the substances that flow easily and they do not have a fixed shape. Liquids and gases are considered as fluids.
They do not have a definite shape and they have weak intermolecular force between their particles. Due to these properties liquids and gases can flow easily.
:
B
Evaporation is a surface phenomenon. The particles of liquid in the wet clothes are always in motion and are loosely packed. They have less force of attraction with each other. These water particles get heated up because of the sun's hot rays, gains enough energy to overcome the force of attraction and gets converted into water vapour by the process of evaporation.
:
B
In the solid state of matter, the particles are much closer to each other and the force of attraction is more between the molecules. Whereas, in a gaseous state of matter, the particles have large space between them and the force of attraction is much lesser when compared to solids and liquids.
:
A
Latent heat of fusion is the heat energy that is absorbed by solid without showing any rise in temperature during phase change at melting point. Since this energy is used for overcoming the inter-particle force of attraction, the kinetic energy of the particles remains constant and hence there is no rise in temperature.
:
A
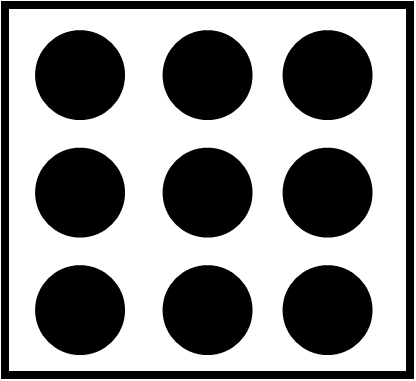
The formula to find density is as follows:
Density=massvolume
The mass of the entire matter inside the given container = 3×9=27 units.
The volume of the given container is 27 units.
Now, density of the container = massvolume = 2727 = 1 unit.
:
A and B
Solids have a tendency to maintain their shape even when an external force is applied. So they have definite shape and volume.
Liquids have a definite volume, but no definite shape. They take the shape of the container.
:
A, C, and D
Particles in solids have lesser kinetic energy when compared to liquids and gases. As temperature increases, the state changes from solids to liquids and then to gases.
As the pressure increases, the volume of the container decreases. As we know, in solids, particles are much closer to each other and the distance between the particles increases as we move towards the gaseous state. So, with the increase in pressure, gases can be converted to liquids and then to solids.
But, change in concentration won't transform matter from one state to another.
:
B
Evaporation causes cooling. The particles of a substance while evaporating absorbs heat energy from its surroundings and convert into vapour. Acetone has a low boiling point of 56∘ C. So, when we put acetone on our hand or skin, being higly volatile, it evaporates rapidly absorbing heat from the hand and leaving back a cold sensation. Therefore, we feel cold.