9th Grade > Chemistry
MATTER IN OUR SURROUNDINGS MCQs
Total Questions : 54
| Page 1 of 6 pages
Answer: Option C. -> sublimation
:
C
Sublimation is the process by which a substance converts directly from its solid to gaseous state. Examples of sublimes are dry ice (solid CO2), camphor, naphthalene, etc.
:
C
Sublimation is the process by which a substance converts directly from its solid to gaseous state. Examples of sublimes are dry ice (solid CO2), camphor, naphthalene, etc.
Answer: Option B. -> Gas
:
B
Compression means reducing the volume. When we compress something, we are decreasingthe volume occupied by it.
When we try to compress a solid, like an iron slab, we would end up either changing the shape or breaking it but we won't really change the volume. This is because the molecules of a solid are very tightly packed. There isn't much space for them to be compressed.
Similarly, if we try to compress a liquid like water it's very difficult to make water occupy a lesser volume. I can't make a litre of water occupy a volume of 500 ml. Of coursethere is more space between the molecules of a liquid, yetit's still difficult to compress it.
But gases are interesting. Gases havelarge spaces between the molecules. We can apply pressure and make a gas occupy a lesser space (volume). This is why we say gases are compressible.
:
B
Compression means reducing the volume. When we compress something, we are decreasingthe volume occupied by it.
When we try to compress a solid, like an iron slab, we would end up either changing the shape or breaking it but we won't really change the volume. This is because the molecules of a solid are very tightly packed. There isn't much space for them to be compressed.
Similarly, if we try to compress a liquid like water it's very difficult to make water occupy a lesser volume. I can't make a litre of water occupy a volume of 500 ml. Of coursethere is more space between the molecules of a liquid, yetit's still difficult to compress it.
But gases are interesting. Gases havelarge spaces between the molecules. We can apply pressure and make a gas occupy a lesser space (volume). This is why we say gases are compressible.
Answer: Option A. -> True
:
A
The energy attained by the particles due to the motion is their kinetic energy. As the temperature increases, the motion of the particles increases and results in higher kinetic energy.
:
A
The energy attained by the particles due to the motion is their kinetic energy. As the temperature increases, the motion of the particles increases and results in higher kinetic energy.
Answer: Option B. -> Matter is particulate
:
B
Matter is considered particulate but not continuous through the experiments like diffusion. For example, a substance like common salt or sugar is able to dissolve in water without any rise in the volume of water. This proves that matter is made up of particles that have space between them to accommodate different types of particles.
:
B
Matter is considered particulate but not continuous through the experiments like diffusion. For example, a substance like common salt or sugar is able to dissolve in water without any rise in the volume of water. This proves that matter is made up of particles that have space between them to accommodate different types of particles.
Answer: Option B. -> False
:
B
Sponge is a solid having tiny pores in it, which traps air. When we try to compress a sponge by applying pressure on it, the air trapped in these holes escape out. Hence, the sponge gets compressed.
However, when the applied pressure is removed, it comes back to its original shape. Hence, even though sponge is compressible, it is a solid.
:
B
Sponge is a solid having tiny pores in it, which traps air. When we try to compress a sponge by applying pressure on it, the air trapped in these holes escape out. Hence, the sponge gets compressed.
However, when the applied pressure is removed, it comes back to its original shape. Hence, even though sponge is compressible, it is a solid.
Answer: Option C. -> Ice at 0∘C
:
C
Ice at0∘Ccan be more effective in coolingbecause of the phenomenon called latent heat of fusion. Latent heat of fusion of ice is theheat absorbed by ice to change its state to water at 0∘C. So, ice at0∘C can extract more heat from another substance and give better cooling.
:
C
Ice at0∘Ccan be more effective in coolingbecause of the phenomenon called latent heat of fusion. Latent heat of fusion of ice is theheat absorbed by ice to change its state to water at 0∘C. So, ice at0∘C can extract more heat from another substance and give better cooling.
Answer: Option B. -> Acetone being volatile, evaporates rapidly and leaves a cooling sensation.
:
B
Evaporation causes cooling. The particles of a substance while evaporating absorbs heat energy from its surroundings and convertinto vapour. Acetone has a lowboiling point of 56∘ C. So, when we put acetone onour hand or skin, being higly volatile,it evaporates rapidly absorbing heatfrom the hand and leaving back a cold sensation.Therefore, we feel cold.
:
B
Evaporation causes cooling. The particles of a substance while evaporating absorbs heat energy from its surroundings and convertinto vapour. Acetone has a lowboiling point of 56∘ C. So, when we put acetone onour hand or skin, being higly volatile,it evaporates rapidly absorbing heatfrom the hand and leaving back a cold sensation.Therefore, we feel cold.
Answer: Option B. -> Evaporation takes place at a temperature below 100∘C.
:
B
Evaporation is the process by which a liquid is converted into vapour at any temperature below the boiling point of the liquid. It is a surface phenomenon. A common example of evaporation is drying of wet clothes.
Whereas, boiling is a bulk phenomenon occuring at a certain temperature called the boiling point. For example, water has a boiling point of 100∘C.
:
B
Evaporation is the process by which a liquid is converted into vapour at any temperature below the boiling point of the liquid. It is a surface phenomenon. A common example of evaporation is drying of wet clothes.
Whereas, boiling is a bulk phenomenon occuring at a certain temperature called the boiling point. For example, water has a boiling point of 100∘C.
Answer: Option A. -> True
:
A
Latent heat of fusion istheheat energy that is absorbed by solid without showing any rise in temperature during phase change at melting point. Since this energy is used for overcoming the inter-particle force of attraction, the kinetic energy of the particles remains constant and hence there is no rise in temperature.
:
A
Latent heat of fusion istheheat energy that is absorbed by solid without showing any rise in temperature during phase change at melting point. Since this energy is used for overcoming the inter-particle force of attraction, the kinetic energy of the particles remains constant and hence there is no rise in temperature.
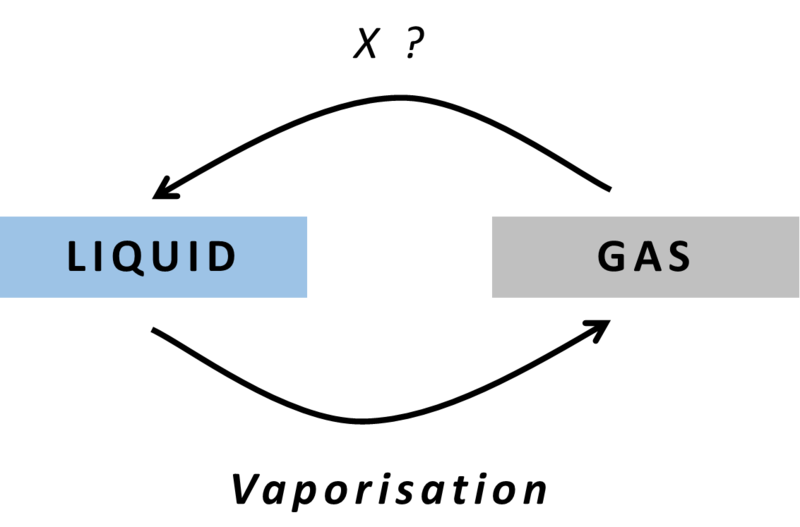
Answer: Option D. -> Condensation
:
D
The particles in a gas have large intermolecular spaces compared to that of a liquid. Hence,gasescan be converted to their liquid state by the process of condensation while releasing energy. It is the reverse of evaporation / vaporization.
:
D
The particles in a gas have large intermolecular spaces compared to that of a liquid. Hence,gasescan be converted to their liquid state by the process of condensation while releasing energy. It is the reverse of evaporation / vaporization.