9th Grade > Chemistry
MATTER IN OUR SURROUNDINGS MCQs
Total Questions : 54
| Page 3 of 6 pages
Answer: Option B. -> False
:
B
In the solid state of matter, the particles are much closer to each other and the force of attraction is more between the molecules. Whereas, in a gaseous state of matter, the particles have large space between them and the force of attraction is much lesser when compared to solids and liquids.
:
B
In the solid state of matter, the particles are much closer to each other and the force of attraction is more between the molecules. Whereas, in a gaseous state of matter, the particles have large space between them and the force of attraction is much lesser when compared to solids and liquids.
Answer: Option B. -> Humidity
:
B
The air around us holdswater in the form ofvapour(gas). Humidity is the amount of water vapour present in the air. During winter, due to low temperature, the humidity in the air falls down. This is the reason why our skin becomes dry in winters.
:
B
The air around us holdswater in the form ofvapour(gas). Humidity is the amount of water vapour present in the air. During winter, due to low temperature, the humidity in the air falls down. This is the reason why our skin becomes dry in winters.
Answer: Option C. -> Gases
:
C
Higher the distance between the particles, lower is the inter particle force of attraction. Hence, particles would be free to move with higher kinetic energy.
Therefore, the order of kinetic energy: Solids < Liquids < Gases
:
C
Higher the distance between the particles, lower is the inter particle force of attraction. Hence, particles would be free to move with higher kinetic energy.
Therefore, the order of kinetic energy: Solids < Liquids < Gases
Answer: Option A. -> 1 unit
:
A
The formula to find density is as follows:
Density=massvolume
The mass of the entire matter inside the given container = 3×9=27units.
The volume of the given container is 27 units.
Now, density of the container = massvolume = 2727 = 1 unit.
:
A
The formula to find density is as follows:
Density=massvolume
The mass of the entire matter inside the given container = 3×9=27units.
The volume of the given container is 27 units.
Now, density of the container = massvolume = 2727 = 1 unit.
Answer: Option A. ->
Matter occupies space.
:
A and B
In this universe, everything that is made up of material is named 'matter'.
All such things that occupy space (volume) and have mass are considered as matter.
The tiny particles that constitute matter cannot be seen with our naked eye.
Matter can be odourless; water and air are two such examples.
:
A and B
In this universe, everything that is made up of material is named 'matter'.
All such things that occupy space (volume) and have mass are considered as matter.
The tiny particles that constitute matter cannot be seen with our naked eye.
Matter can be odourless; water and air are two such examples.
Answer: Option B. ->
Matter is particulate
:
B
Matter is considered particulate but not continuous through the experiments like diffusion. For example, a substance like common salt or sugar is able to dissolve in water without any rise in the volume of water. This proves that matter is made up of particles that have space between them to accommodate different types of particles.
:
B
Matter is considered particulate but not continuous through the experiments like diffusion. For example, a substance like common salt or sugar is able to dissolve in water without any rise in the volume of water. This proves that matter is made up of particles that have space between them to accommodate different types of particles.
Answer: Option C. ->
Iron
:
C
As we know, more the distance between the particles, lesser is the force of attraction between them. Nitrogen is a gas at room temperature. So, its particles have more space and less attraction between them than a solid.
Mercury is liquid at room temperature. So, its particles have comparatively more space and less attraction between them, than a solid.
Chalk piece can be easily broken because its particles have weaker force of attraction than that of iron.
Hence, the correct order of force of attraction is as follows:
Nitrogen < Mercury < Chalk < Iron
:
C
As we know, more the distance between the particles, lesser is the force of attraction between them. Nitrogen is a gas at room temperature. So, its particles have more space and less attraction between them than a solid.
Mercury is liquid at room temperature. So, its particles have comparatively more space and less attraction between them, than a solid.
Chalk piece can be easily broken because its particles have weaker force of attraction than that of iron.
Hence, the correct order of force of attraction is as follows:
Nitrogen < Mercury < Chalk < Iron
Answer: Option C. ->
Particles of matter have comparable size as that of the matter.
:
A, B, and D
The matter is made of discrete particles.
1. The particles can accommodate different particles as they have space between them. Example: Sugar dissolving in water without any change in volume of the mixture.
2. The particles are in continuous motion. They do not have fixed position. This is the reason why the gas molecules hit the walls of a container and exert pressure. The particles in a solid are in motion too.
3. The particles are very small as compared to the matter.
4. The particles attract each other. This is the reason why liquid water has to be heated to break the bonds between water molecules and to convert them into vapour.
:
A, B, and D
The matter is made of discrete particles.
1. The particles can accommodate different particles as they have space between them. Example: Sugar dissolving in water without any change in volume of the mixture.
2. The particles are in continuous motion. They do not have fixed position. This is the reason why the gas molecules hit the walls of a container and exert pressure. The particles in a solid are in motion too.
3. The particles are very small as compared to the matter.
4. The particles attract each other. This is the reason why liquid water has to be heated to break the bonds between water molecules and to convert them into vapour.
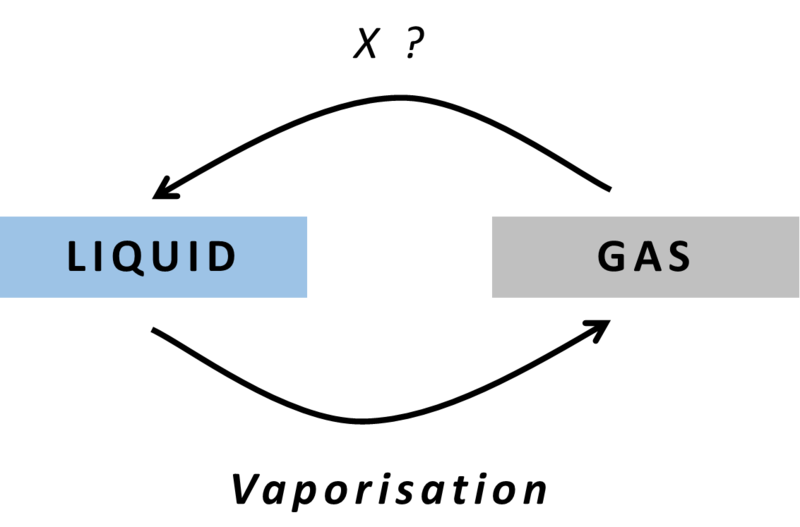
Answer: Option D. ->
Condensation
:
D
The particles in a gas have large intermolecular spaces compared to that of a liquid. Hence, gases can be converted to their liquid state by the process of condensation while releasing energy. It is the reverse of evaporation / vaporization.
:
D
The particles in a gas have large intermolecular spaces compared to that of a liquid. Hence, gases can be converted to their liquid state by the process of condensation while releasing energy. It is the reverse of evaporation / vaporization.
Answer: Option A. ->
True
:
A
The energy attained by the particles due to the motion is their kinetic energy. As the temperature increases, the motion of the particles increases and results in higher kinetic energy.
:
A
The energy attained by the particles due to the motion is their kinetic energy. As the temperature increases, the motion of the particles increases and results in higher kinetic energy.