10th Grade > Chemistry
CARBON AND ITS COMPOUNDS MCQs
Total Questions : 56
| Page 5 of 6 pages
Answer: Option D. ->
ketone
:
D
The given compound is propanone having the formula CH3COCH3. The functional group present in this compound is ketone as it has C=O group present in between two alkyl groups.
:
D
The given compound is propanone having the formula CH3COCH3. The functional group present in this compound is ketone as it has C=O group present in between two alkyl groups.
Answer: Option B. ->
ethene
:
B
In the given situation, concentrated sulphuric acid act as a dehydrating agent.
When ethanol is heated at 443 K with excess concentrated sulphuric acid, it causes its dehydration (loss of water). This results in the formation of ethene.
The reaction occurs as follows:
CH3CH2OH(aq)Hot Conc.−−−−−−→H2SO4CH2=CH2(g)+H2O(l)
:
B
In the given situation, concentrated sulphuric acid act as a dehydrating agent.
When ethanol is heated at 443 K with excess concentrated sulphuric acid, it causes its dehydration (loss of water). This results in the formation of ethene.
The reaction occurs as follows:
CH3CH2OH(aq)Hot Conc.−−−−−−→H2SO4CH2=CH2(g)+H2O(l)
Answer: Option C. ->
Methanol and propanoic acid
:
C
:
C
Esters are obtained from the reaction between carboxylic acid and alcohols. The reaction between methanol and propanoic acid in the presence of concentrated sulphuric acid leads to the formation of methyl propanoate, an ester.
CH3OH(aq)+CH3CH2COOH(aq)→CH3CH2COOCH3(aq)+H2O(l)
Answer: Option C. ->
but-2-yne
:
C
The given compound can be numbered as shown below.
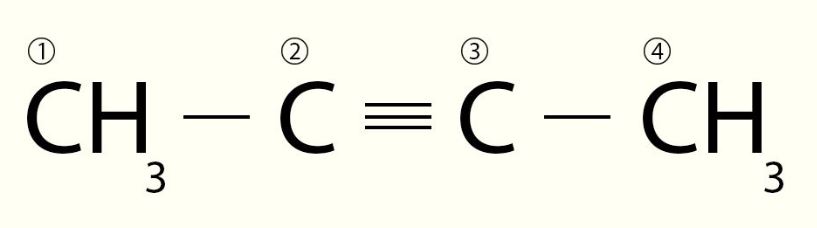
∙ There are four carbon atoms in the parent chain (the longest carbon chain) and hence, the root word is 'but'.
∙ A triple bond is present between the second and third carbon atoms of the chain. Hence, it is an alkyne and the suffix will be '2-yne'.
:
C
The given compound can be numbered as shown below.
∙ There are four carbon atoms in the parent chain (the longest carbon chain) and hence, the root word is 'but'.
∙ A triple bond is present between the second and third carbon atoms of the chain. Hence, it is an alkyne and the suffix will be '2-yne'.
Therefore, the IUPAC name of the given compound is but-2-yne.
Answer: Option C. ->
Valency of the heteroatom
:
A, B, and D
The points to be considered while writing the IUPAC names are:
-The number of carbon atoms in the parent chain, which is the longest continuous chain in a compound. This would give the root word of the compound.
- Presence or absence of unsaturation in carbon chain, determines the primary suffix of the compound i.e. whether the compound is alkane, alkene or alkyne.
- The functional group present as it can be an alcohol, an aldehyde, a ketone, etc. According to the functional group present, the secondary suffix in the name of the compound changes.
- Heteroatoms are those atoms, other than carbon and hydrogen atoms, which are attached to one or more carbon atoms in an organic compound. However, the valency of these atoms are not considered while naming an organic compound.
:
A, B, and D
The points to be considered while writing the IUPAC names are:
-The number of carbon atoms in the parent chain, which is the longest continuous chain in a compound. This would give the root word of the compound.
- Presence or absence of unsaturation in carbon chain, determines the primary suffix of the compound i.e. whether the compound is alkane, alkene or alkyne.
- The functional group present as it can be an alcohol, an aldehyde, a ketone, etc. According to the functional group present, the secondary suffix in the name of the compound changes.
- Heteroatoms are those atoms, other than carbon and hydrogen atoms, which are attached to one or more carbon atoms in an organic compound. However, the valency of these atoms are not considered while naming an organic compound.
Answer: Option B. ->
C2H2
:
B and C
The reaction in which atoms combine with unsaturated carbon compounds to form saturated carbon compounds is called addition reaction. Butane and propane are saturated hydrocarbons and hence, they will not undergo addition reaction while propene and acetylene are unsaturated hydrocarbons which will undergo addition reaction.
For example, propene will undergo an addition reaction with chlorine to form 1, 2, Dichloropropane. CH3CH=CH2(g)+Cl2(g)→CH3CHClCH2Cl(g)
Acetylene will undergo an addition reaction with hydrogen to form ethene.
CH≡CH(g)+H2→CH2=CH2(g)
:
B and C
The reaction in which atoms combine with unsaturated carbon compounds to form saturated carbon compounds is called addition reaction. Butane and propane are saturated hydrocarbons and hence, they will not undergo addition reaction while propene and acetylene are unsaturated hydrocarbons which will undergo addition reaction.
For example, propene will undergo an addition reaction with chlorine to form 1, 2, Dichloropropane. CH3CH=CH2(g)+Cl2(g)→CH3CHClCH2Cl(g)
Acetylene will undergo an addition reaction with hydrogen to form ethene.
CH≡CH(g)+H2→CH2=CH2(g)
Answer: Option B. ->
Two
:
B
Ethyne (C2H2) has two carbon-hydrogen single bonds and one carbon-carbon triple bond.
The structure of ethyne is H−C≡C−H.
:
B
Ethyne (C2H2) has two carbon-hydrogen single bonds and one carbon-carbon triple bond.
The structure of ethyne is H−C≡C−H.
Answer: Option A. ->
Methanol
:
A
Methanol (CH3OH) is added to denature ethanol.
Denatured alcohol, also called methylated spirits has additives to make it poisonous, extremely bad tasting, foul smelling or nauseating. This is done in order to discourage its recreational consumption.
:
A
Methanol (CH3OH) is added to denature ethanol.
Denatured alcohol, also called methylated spirits has additives to make it poisonous, extremely bad tasting, foul smelling or nauseating. This is done in order to discourage its recreational consumption.
Answer: Option C. ->
2Na + 2CH3CH2OH →2CH3CH2ONa + H2
:
C
The reaction between sodium and alcohol is a metal-acid reaction. Metals generally liberate hydrogen on reacting with acids.
Ethanol reacts with sodium leading to the evolution of hydrogen and sodium ethoxide. The reaction occurs as follows:
2Na(s) + 2CH3CH2OH(aq) →2CH3CH2ONa(aq) + H2(g)
:
C
The reaction between sodium and alcohol is a metal-acid reaction. Metals generally liberate hydrogen on reacting with acids.
Ethanol reacts with sodium leading to the evolution of hydrogen and sodium ethoxide. The reaction occurs as follows:
2Na(s) + 2CH3CH2OH(aq) →2CH3CH2ONa(aq) + H2(g)
Answer: Option B. ->
Saturated compounds- clean or blue flame, Unsaturated compounds - yellow flame.
:
B
:
B
- Saturated hydrocarbons have a low percentage composition of carbon in them. So, the atmospheric oxygen is enough to burn all the carbon present. Therefore, these compounds give clean or blue flame.
- In unsaturated carbons, the percentage composition of carbon is high and the atmospheric oxygen is not enough to burn all the carbon present. The yellow flame is caused by the glow of hot unburnt carbon particles produced due to incomplete combustion..
















