11th And 12th > Physics
X - RAYS MCQs
Total Questions : 15
| Page 1 of 2 pages
Answer: Option B. ->
X-Rays are highly energetic, as compared to visible light.
:
B
X-rays are a part of electromagnetic spectrum and they fall in the high energy region of it.
They are not radioactive neither charged, but it is only them being highly energetic that they may affect the chemical bonds inside the body and change certain mechanisms if not destroy them (simply speaking creating mutation in fundamental building blocks of human body as D.N.A.). Medical science calls these types of mutations "Cancer".
That’s why they are harmful.
But don't get scared of becoming some mutilated character next time when you go for an x-ray examination!! The chances of this are very less and the benefits are definitely more (your doctor gets to see your fractures). The cancer may be caused only due to excess exposure to direct radiation.
:
B
X-rays are a part of electromagnetic spectrum and they fall in the high energy region of it.
They are not radioactive neither charged, but it is only them being highly energetic that they may affect the chemical bonds inside the body and change certain mechanisms if not destroy them (simply speaking creating mutation in fundamental building blocks of human body as D.N.A.). Medical science calls these types of mutations "Cancer".
That’s why they are harmful.
But don't get scared of becoming some mutilated character next time when you go for an x-ray examination!! The chances of this are very less and the benefits are definitely more (your doctor gets to see your fractures). The cancer may be caused only due to excess exposure to direct radiation.
Answer: Option B. ->
Magnetic field would not affect the x-rays as it is not a charged particle
:
B and D
Magnetic field can only influence a magnet and hence moving charges (Because they are magnets too). x-ray on the other hand is not a charged particle but a packet of electro-magnetic energy. Hence bending x-rays using a magnetic field is out of the equation, moreover even if tried so, a presence of magnetic field could affect the path of electrons present in the tube. Won't it ?
:
B and D
Magnetic field can only influence a magnet and hence moving charges (Because they are magnets too). x-ray on the other hand is not a charged particle but a packet of electro-magnetic energy. Hence bending x-rays using a magnetic field is out of the equation, moreover even if tried so, a presence of magnetic field could affect the path of electrons present in the tube. Won't it ?
Answer: Option B. ->
5.82 keV
:
B
In our notation of energy levels, an energy EX will mean the energy of the atom when there's a missing electron in the X shell ( X = K, L, M, N,....). Writing quantitatively.
E(Kα) = EK−EL
⇒EL=EK−E(Kα)
⇒EL=EK−(hcλKα)
⇒EL=23.32keV−(1242eV.nm0.071nm)=23.32keV−17.5eV
⇒EL=5.82keV.
:
B
In our notation of energy levels, an energy EX will mean the energy of the atom when there's a missing electron in the X shell ( X = K, L, M, N,....). Writing quantitatively.
E(Kα) = EK−EL
⇒EL=EK−E(Kα)
⇒EL=EK−(hcλKα)
⇒EL=23.32keV−(1242eV.nm0.071nm)=23.32keV−17.5eV
⇒EL=5.82keV.
Answer: Option B. ->
M→L
:
B and C
So we have an electronic vacancy created in L-shell. Now let's think about each cases one by one. We just have to keep in mind that every system acts in a way to minimize its energy. No process is spontaneously possible in which the energy of the system increases. Only those transition/s will be possible after which the energy of the atom decreases.
From the atomic energy level diagram we know that the atomic energy is highest when there is a vacancy in K-shell and decreases further. i.e.
EK > EL > EM > ENAnd so on.
If a transition from K-shell occurs, the vacancy will shift to K-shell. The atomic energy increase hence. So, this is not possible.
If a transition from M-shell happens, the atomic energy decreases, hence such a transition is possible.
Infact any transition in which the electron is jumping from a shell above L-shell to fill a vacancy here is possible.
:
B and C
So we have an electronic vacancy created in L-shell. Now let's think about each cases one by one. We just have to keep in mind that every system acts in a way to minimize its energy. No process is spontaneously possible in which the energy of the system increases. Only those transition/s will be possible after which the energy of the atom decreases.
From the atomic energy level diagram we know that the atomic energy is highest when there is a vacancy in K-shell and decreases further. i.e.
EK > EL > EM > ENAnd so on.
If a transition from K-shell occurs, the vacancy will shift to K-shell. The atomic energy increase hence. So, this is not possible.
If a transition from M-shell happens, the atomic energy decreases, hence such a transition is possible.
Infact any transition in which the electron is jumping from a shell above L-shell to fill a vacancy here is possible.
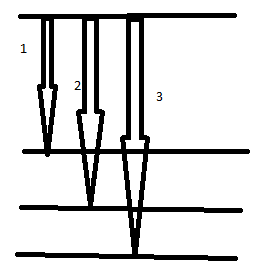
Answer: Option A. ->
1-x, 2-y, 3-z
:
A
Kα : When electronis transition takes place from L-shell to K-shell or vacancy shifts from K-shell to L-shell (this is what the energy level diagram indicates)
Kβ : When electronis transition takes place from M-shell to K-shell or vacancy shifts from K-shell to M-shell (this is what the energy level diagram indicates)
Kγ : When electronis transition takes place from N-shell to K-shell or vacancy shifts from K-shell to N-shell (this is what the energy level diagram indicates)
Got the answer!!
:
A
This is a definition based question.
We defined as following :
Kα : When electronis transition takes place from L-shell to K-shell or vacancy shifts from K-shell to L-shell (this is what the energy level diagram indicates)
Kβ : When electronis transition takes place from M-shell to K-shell or vacancy shifts from K-shell to M-shell (this is what the energy level diagram indicates)
Kγ : When electronis transition takes place from N-shell to K-shell or vacancy shifts from K-shell to N-shell (this is what the energy level diagram indicates)
Got the answer!!
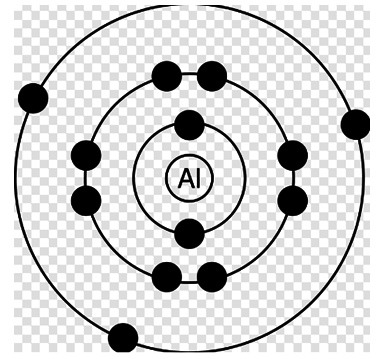
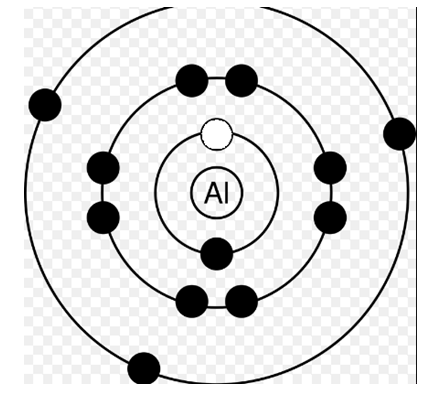
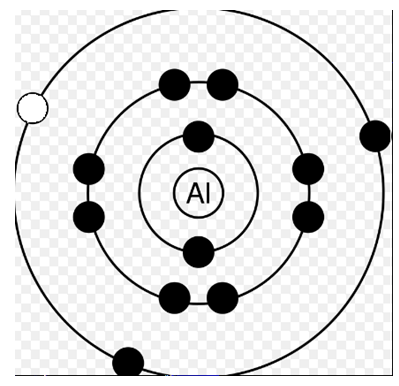
Answer: Option D. ->
II > IV > III > I
:
D
It’s a very simple question. We have to compare the energy in all the four diagrams. Basically these diagrams represent an atom with different electronic configuration with, infact, an electron missing from a specific shell in each case. Proceeding organically, first of all let's say the energy in the first case (with each electron at its place) is x.
Now, in the second diagram the electron is removed from the K-shell. We need to supply energy to the atom to do this right? So let's say we supplied an energy ΔK.
So, the atom's energy now becomes x+ΔK.
Now in the third diagram the electron is removed from M shell. We need to supply energy even for that. Let's say that energy is ΔM.
So, atom’s energy becomes x+ΔM.
In the fourth diagram, similarly after removing electron from L-shell the atomic energy becomes x+ΔL.
Now, we understand that it is tougher to remove the electron which is closer to the nucleus as it is more strongly bounded with the nucleus. To remove the electron from K-shell we need to supply the highest amount of energy, and to remove an electron from the M-shell we need to supply the least amount of energy. Mathematically,ΔK > ΔL > ΔM
Hence the energy of the atom will be in the order: II > IV > III > I.
:
D
It’s a very simple question. We have to compare the energy in all the four diagrams. Basically these diagrams represent an atom with different electronic configuration with, infact, an electron missing from a specific shell in each case. Proceeding organically, first of all let's say the energy in the first case (with each electron at its place) is x.
Now, in the second diagram the electron is removed from the K-shell. We need to supply energy to the atom to do this right? So let's say we supplied an energy ΔK.
So, the atom's energy now becomes x+ΔK.
Now in the third diagram the electron is removed from M shell. We need to supply energy even for that. Let's say that energy is ΔM.
So, atom’s energy becomes x+ΔM.
In the fourth diagram, similarly after removing electron from L-shell the atomic energy becomes x+ΔL.
Now, we understand that it is tougher to remove the electron which is closer to the nucleus as it is more strongly bounded with the nucleus. To remove the electron from K-shell we need to supply the highest amount of energy, and to remove an electron from the M-shell we need to supply the least amount of energy. Mathematically,ΔK > ΔL > ΔM
Hence the energy of the atom will be in the order: II > IV > III > I.
Answer: Option A. ->
0.59 MeV
:
A
Kα x-ray emission takes place when an electron jumps from L-shell to fill a vacancy created in K-shell.
Now EK is the energy of the atom when vacancy is in K-Shell, and EL Is the energy of the atom when vacancy is created in L-Shell. Also, EK > EL So, when vacancy shifts from K to L, according to our agrument, the atomic energy decreases. How much ?
EK−EL.
This difference results into X-ray photon.
Hence connecting the dots we can conclude that ; The energy difference between K and L levels is the energy of the photon.
Mathematically;
EK−EL=Ephoton
Now,
EK−EL=hcλ
Which gives after putting in the values;
EK−EL=1242 eVnm0.0021nm
Final calculations leads us to our answer;
EK−EL=591428eV
Or,
EK−EL=0.59MeV
:
A
Kα x-ray emission takes place when an electron jumps from L-shell to fill a vacancy created in K-shell.
Now EK is the energy of the atom when vacancy is in K-Shell, and EL Is the energy of the atom when vacancy is created in L-Shell. Also, EK > EL So, when vacancy shifts from K to L, according to our agrument, the atomic energy decreases. How much ?
EK−EL.
This difference results into X-ray photon.
Hence connecting the dots we can conclude that ; The energy difference between K and L levels is the energy of the photon.
Mathematically;
EK−EL=Ephoton
Now,
EK−EL=hcλ
Which gives after putting in the values;
EK−EL=1242 eVnm0.0021nm
Final calculations leads us to our answer;
EK−EL=591428eV
Or,
EK−EL=0.59MeV
Answer: Option A. ->
hcEK−EM
:
A
Kβ characteristic X-Ray phton is emitted when an electron from the M shell jumps to fill the electronic vacancy created in K shell.
Now, if we refer to the Energy Level Diagram, we find that The atomic energy when there is a vacancy in K shell is EK.
Also the atomic energy when the vacancy gets created in M shell is EM.
We see that EM < EK.
So the atom has lost this energy. How much ?
EK−EM
This energy appears as the energy of Kβ characteristic X-Ray phton.
Hence hcλKβ=EK−EM.
Which gives,
1λKβ=EK−EM.
:
A
Kβ characteristic X-Ray phton is emitted when an electron from the M shell jumps to fill the electronic vacancy created in K shell.
Now, if we refer to the Energy Level Diagram, we find that The atomic energy when there is a vacancy in K shell is EK.
Also the atomic energy when the vacancy gets created in M shell is EM.
We see that EM < EK.
So the atom has lost this energy. How much ?
EK−EM
This energy appears as the energy of Kβ characteristic X-Ray phton.
Hence hcλKβ=EK−EM.
Which gives,
1λKβ=EK−EM.
Answer: Option C. ->
17.5 pm
:
C
For the wavelength of Kα x-rays; hcλ1 = EK−EL
for the wavelength of Lα x-rays; hcλ2 = EL−EM
or the wavelength of Kβ x-rays; hcλ3 = EK−EM
Now,
If we add the first two equation we get :
hc(1λ1+1λ2)=EK−EM
Now from equations iii) and iv)
hcλ3=hc(1λ1+1λ2).
We get;
1λ3=(1λ1+1λ2).
Or,
1λ3=(120+1140)pm−1
Or,
λ3=2800160pm.
Or,
λ3 = 17.5 pm
:
C
For the wavelength of Kα x-rays; hcλ1 = EK−EL
for the wavelength of Lα x-rays; hcλ2 = EL−EM
or the wavelength of Kβ x-rays; hcλ3 = EK−EM
Now,
If we add the first two equation we get :
hc(1λ1+1λ2)=EK−EM
Now from equations iii) and iv)
hcλ3=hc(1λ1+1λ2).
We get;
1λ3=(1λ1+1λ2).
Or,
1λ3=(120+1140)pm−1
Or,
λ3=2800160pm.
Or,
λ3 = 17.5 pm
Answer: Option B. ->
The energy of atom when electron is removed from that particular shell
:
B
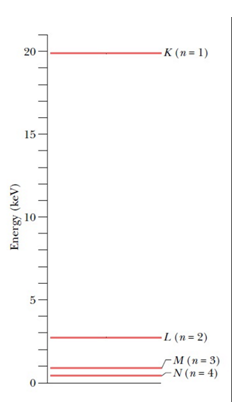
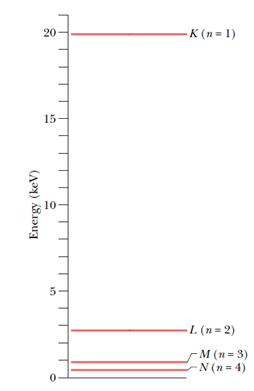
The energies marked in the diagram are the energies of the atom when a vacancy of electron is created in particular shells.
For example, the energy marked as EK is the energy of atom when one electron from the K shell is removed. And so on.
It is interesting to note that the energy of the atom with one vacancy in K shell is higher; and as we move towards the higher shells, the energies of the atom with vacancies in those shells decrease. Also, the energy is zero when the atom is in its ground state.
This can be understood simply, as the K shell electrons are bound to the nucleus most strongly; this force decreases as we move to higher shells. Hence, to remove an electron from the lowermost shell (K) maximum amount of energy is required to be supplied, this amount will decrease as we move towards higher shells; for the same reason.
In the ground state (no electrons removed) the atom’s energy will be the least, which is taken to be zero.
:
B

The energies marked in the diagram are the energies of the atom when a vacancy of electron is created in particular shells.
For example, the energy marked as EK is the energy of atom when one electron from the K shell is removed. And so on.
It is interesting to note that the energy of the atom with one vacancy in K shell is higher; and as we move towards the higher shells, the energies of the atom with vacancies in those shells decrease. Also, the energy is zero when the atom is in its ground state.
This can be understood simply, as the K shell electrons are bound to the nucleus most strongly; this force decreases as we move to higher shells. Hence, to remove an electron from the lowermost shell (K) maximum amount of energy is required to be supplied, this amount will decrease as we move towards higher shells; for the same reason.
In the ground state (no electrons removed) the atom’s energy will be the least, which is taken to be zero.