11th And 12th > Chemistry
STEREOCHEMISTRY MCQs
Total Questions : 15
| Page 1 of 2 pages
Answer: Option A. ->
Nonsuperimposable on its mirror image
:
A, B, C, and D
First, look at the central carbon. Is it sp3 hybridized? Definitely. Can you recall the definition of a chiral center?
A chiral center is any sp3 hybridized atom attached to 4 different groups. One notable exception is a tertiary amine which undergoes rapid flipping and hence cannot be categorized as a chiral center.
Also, there are four different groups attached to the central Carbon atom. Hence, it is a chiral center. But does that make the molecule chiral?
What is a chiral molecule? A molecule whose image is not superimposable on the original is called chiral. Such molecules rotate plane polarized light and are called optically active. Do note that if a compound has only one center, it usually tends to be chiral. But the presence of a chiral center alone is neither a necessary nor a sufficient condition for the molecule to be called chiral. Let us do the mirror test:
If we try to superimpose the F - C - Cl bonds, we see that the other elements do not coincide. In other words, there is a lateral inversion and this makes the mirror image non-superimposable on top of the original (to the left). The image and the original are non-identical. Hence all the options are correct.
:
A, B, C, and D
First, look at the central carbon. Is it sp3 hybridized? Definitely. Can you recall the definition of a chiral center?
A chiral center is any sp3 hybridized atom attached to 4 different groups. One notable exception is a tertiary amine which undergoes rapid flipping and hence cannot be categorized as a chiral center.
Also, there are four different groups attached to the central Carbon atom. Hence, it is a chiral center. But does that make the molecule chiral?
What is a chiral molecule? A molecule whose image is not superimposable on the original is called chiral. Such molecules rotate plane polarized light and are called optically active. Do note that if a compound has only one center, it usually tends to be chiral. But the presence of a chiral center alone is neither a necessary nor a sufficient condition for the molecule to be called chiral. Let us do the mirror test:

If we try to superimpose the F - C - Cl bonds, we see that the other elements do not coincide. In other words, there is a lateral inversion and this makes the mirror image non-superimposable on top of the original (to the left). The image and the original are non-identical. Hence all the options are correct.
Answer: Option A. ->
Dextrorotatory
:
A
:
A
We usually call compounds which can rotate compounds in the clockwise direction as Dextrorotatory.
Similarly, the compounds which can rotate light in the anticlockwise direction are called Laevorotatory.
If you are confused whether to use R/S or D/L here, let me clear that for you. R/S nomenclature is used to denote the way in which the molecules in the compounds are oriented in space which helps us differentiate between stereoisomers in a simple and effective way. It has nothing to do with the way it can rotate light.
An R molecule may be either D/L. There is no link between R/S and D/L.
Please don't forget this!
Answer: Option A. ->
Dextrorotatory
:
When we have a 50:50 mixture of a pair of enantiomers, it is called a racemic mixture or recemate. A racemic mixture does not show any optical activity although it is made up of two enantiomers which are optically active.
If we have a mixture in any other ratio, then the resultant sample is always optically active. In this scenario it is said that there is an enantiomeric excess.
Enantiomeric excess is the percentage or ratio by which one enantiomer is greater in quantity than the other enantiomer present in the same sample.
Since there is no difference between the quantities of the two enantiomers present in a racemic solution, the enantiomeric excess is '0'.
:
When we have a 50:50 mixture of a pair of enantiomers, it is called a racemic mixture or recemate. A racemic mixture does not show any optical activity although it is made up of two enantiomers which are optically active.
If we have a mixture in any other ratio, then the resultant sample is always optically active. In this scenario it is said that there is an enantiomeric excess.
Enantiomeric excess is the percentage or ratio by which one enantiomer is greater in quantity than the other enantiomer present in the same sample.
Since there is no difference between the quantities of the two enantiomers present in a racemic solution, the enantiomeric excess is '0'.
Answer: Option B. ->
False
:
B
Looks tricky doesn’t it?
Let us run through the definition of a meso compound. It has to have at least 2 tetrahedral stereogenic centers. Further, it has to be achiral due to a plane of symmetry.
Let us see if we can fit the given compound into the above framework.
trans-1,3-dichlorocyclobutane (Chlorine atoms are in green; Carbon in grey)

Can you think of a plane that bisects the molecule into two mirror images of each other? As you can see, the 4 carbons are not planar – and the Chlorine atoms are on the opposite sides. Let us shift to a different view:

It becomes immediately clear that the 4 carbons are not in a plane. It is almost as if the compound is like:
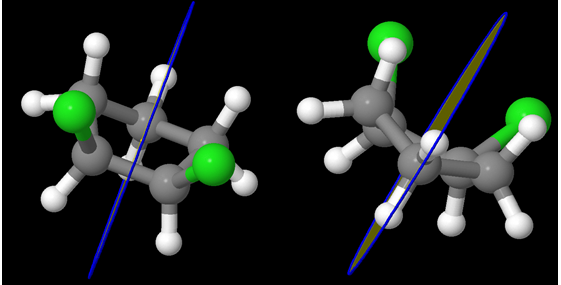
One Chlorine is axial and the other is equatorial! But the question remains – id there a plane of symmetry?
Interestingly, there is one isn’t there?
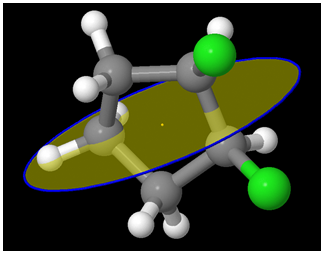
A different view:
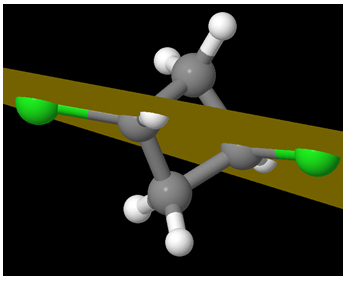
A view from another perspective:
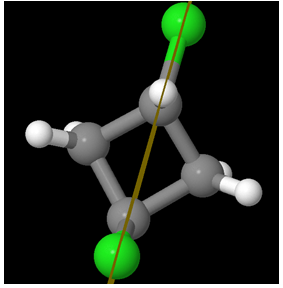
So the molecule is Achiral. But hey! Are there at least two chiral centers?
Ha Ha! There are NO chiral centers!
You might be tempted to think there are – but there aren’t!
Look at the carbon atoms attached to the Chlorine atoms. They are attached to two equivalent (CH2) groups. In the above diagram, start from the Carbon at the top. As we go through the left part of the cyclic compound, we first encounter a single bon CH2 and then reach a CHCl, followed by a CH2 group again and finish with the topmost carbon.
Do the same exercise but in the right part of the chain (clockwise). Start with the carbon at the top. Go right to the single bond CH2 group. From there we find a CHCl group and then again a CH2 group and then finish with the topmost carbon! So there is no point of difference!
Hence, there are NO chiral centers for trans-1,3-dichlorocyclobutane. Thus it is not a meso compound.
:
B
Looks tricky doesn’t it?
Let us run through the definition of a meso compound. It has to have at least 2 tetrahedral stereogenic centers. Further, it has to be achiral due to a plane of symmetry.
Let us see if we can fit the given compound into the above framework.
trans-1,3-dichlorocyclobutane (Chlorine atoms are in green; Carbon in grey)

Can you think of a plane that bisects the molecule into two mirror images of each other? As you can see, the 4 carbons are not planar – and the Chlorine atoms are on the opposite sides. Let us shift to a different view:

It becomes immediately clear that the 4 carbons are not in a plane. It is almost as if the compound is like:

One Chlorine is axial and the other is equatorial! But the question remains – id there a plane of symmetry?
Interestingly, there is one isn’t there?

A different view:

A view from another perspective:

So the molecule is Achiral. But hey! Are there at least two chiral centers?
Ha Ha! There are NO chiral centers!
You might be tempted to think there are – but there aren’t!
Look at the carbon atoms attached to the Chlorine atoms. They are attached to two equivalent (CH2) groups. In the above diagram, start from the Carbon at the top. As we go through the left part of the cyclic compound, we first encounter a single bon CH2 and then reach a CHCl, followed by a CH2 group again and finish with the topmost carbon.
Do the same exercise but in the right part of the chain (clockwise). Start with the carbon at the top. Go right to the single bond CH2 group. From there we find a CHCl group and then again a CH2 group and then finish with the topmost carbon! So there is no point of difference!
Hence, there are NO chiral centers for trans-1,3-dichlorocyclobutane. Thus it is not a meso compound.
Answer: Option B. ->
Nicol
:
B
The type of prism used in Biot’s experiment is called a Nicol prism. This is a special material which is able to polarise light passing through it.
You know that light normally vibrates in all possible planes when coming out of a source. But when passed through special devices like this Nicol prism it comes out vibrating in just one direction. This allows us to test for optical activity.
:
B
The type of prism used in Biot’s experiment is called a Nicol prism. This is a special material which is able to polarise light passing through it.
You know that light normally vibrates in all possible planes when coming out of a source. But when passed through special devices like this Nicol prism it comes out vibrating in just one direction. This allows us to test for optical activity.
Answer: Option B. ->

:
B and C
In the previous question, we saw that the first compound

Is not meso. Why? It does not have any chiral centers!
What about the following compound?

It is a tetra-substituted n butane but shown in staggered configuration. Do remember that although the staggered configuration shown is the most stable one, we can rotate the molecule any which way we please. Let us ty and rotate the molecule about the C2 – C3 single bond to get the eclipsed (most unstable) conformer. It looks like:

Now, it looks pretty symmetric doesn’t it? Can you visualize a plane that bisects the molecule into two equal symmetrical halves? It will be perpendicular to the C2 – C3 single bond.
Are the carbons C2 and C3 chiral? They are – aren’t they? Each one is attached to four different groups – Bromine, chlorine, methyl and bromochloromethyl groups!
This is definitely meso.
Similarly, cis-1,2-dichlorocyclopentane is also meso. It has two chiral carbons each having a Chlorine substituent. Check for yourself why they are chiral. A plane that passes through one of the carbons and perpendicularly bisecting the bond between C1 and C2 is a plane of symmetry.

The plane of symmetry is:


The trans isomer is not meso. Why? Simply because it is a chiral molecule! Do remember that the meso compound should have two or more tetrahedral chiral centers but still should be achiral!

:
B and C
In the previous question, we saw that the first compound

Is not meso. Why? It does not have any chiral centers!
What about the following compound?

It is a tetra-substituted n butane but shown in staggered configuration. Do remember that although the staggered configuration shown is the most stable one, we can rotate the molecule any which way we please. Let us ty and rotate the molecule about the C2 – C3 single bond to get the eclipsed (most unstable) conformer. It looks like:

Now, it looks pretty symmetric doesn’t it? Can you visualize a plane that bisects the molecule into two equal symmetrical halves? It will be perpendicular to the C2 – C3 single bond.
Are the carbons C2 and C3 chiral? They are – aren’t they? Each one is attached to four different groups – Bromine, chlorine, methyl and bromochloromethyl groups!
This is definitely meso.
Similarly, cis-1,2-dichlorocyclopentane is also meso. It has two chiral carbons each having a Chlorine substituent. Check for yourself why they are chiral. A plane that passes through one of the carbons and perpendicularly bisecting the bond between C1 and C2 is a plane of symmetry.

The plane of symmetry is:


The trans isomer is not meso. Why? Simply because it is a chiral molecule! Do remember that the meso compound should have two or more tetrahedral chiral centers but still should be achiral!
Answer: Option B. ->
−CH(CH3)2 > −CH2CH2CH3 > - D > - H
:
First step while writing the R - S nomenclature is to identify the atoms and their atomic number.
Here we have - H - 1
F - 9
Cl - 17
Br - 35
Now, we need to place the order of priority accordingly. So, the order of priority would be
Br > Cl > F > H
Now, we need to place the atom with the lowest priority at the back, i.e., facing away from you when you look at the carbon. Let’s do that now.

Here we have H above the plane and F below the plane.
If I need H to be away, I need to look at this molecule in this way.

I hope you can visualize this.
Let’s draw this like this and number it.

Another way is to convert this to Fischer projection. You will learn that soon.
:
First step while writing the R - S nomenclature is to identify the atoms and their atomic number.
Here we have - H - 1
F - 9
Cl - 17
Br - 35
Now, we need to place the order of priority accordingly. So, the order of priority would be
Br > Cl > F > H
Now, we need to place the atom with the lowest priority at the back, i.e., facing away from you when you look at the carbon. Let’s do that now.

Here we have H above the plane and F below the plane.
If I need H to be away, I need to look at this molecule in this way.

I hope you can visualize this.
Let’s draw this like this and number it.

Another way is to convert this to Fischer projection. You will learn that soon.