11th And 12th > Chemistry
SOLID STATE MCQs
:
C
Following are the characteristic features of a solid state:
I. Definite shape, volume and mass
II. Intermolecular distances are short and intermolecular forces are strong
III. They are incompressible and rigid
IV. The constituent particles have fixed positions and can oscillate only about their mean positions
:
B
Amorphous solids consist of particles of irregular shape, so it displays short range order.
Amorphous solids soften over a range of temperature, and hence doesn’t show sharp melting point.
The value of a physical property will be same from any directions in case of amorphous solids. So, they are said to be isotropic in nature.
Amorphous solids do not have a definite heat of fusion.
:
D
Polar and non-polar molecular solids are soft and non-conductors of electricity.
Ionic solids are non-conductors of electricity in solid state, as the constituent ions are not free to move (but in molten state or when dissolved in water the ions are free to move and hence they conduct electricity)
:
C
Choose the correct option:
Crystal system Axial distanceAxial angles(a)Tetragonala=b≠cα=β=γ=90∘(b)Monoclinica≠b≠cα=γ=90∘β≠90∘(c)Rhombohedrala = b = cα=β=γ≠90∘(d)Triclinica≠b≠cα≠β≠γ≠90∘
:
D
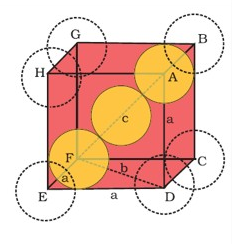
The bcc cell consistes of 8 atoms at the corners and one atom at centre.
∴n=(8×18)+1=2.
The fcc cell consists of 8 atoms at the eight corners and one atom at each of the six faces.This atom at the faces is shared by two unit cells.
∴n=8×18+(6×12)=4
:
D
In face-centered cubic arrangement, 74% of the crystal space is filled
- Vacant space = 100 – 74 = 26%
:
C
Tetrahedral sites are double compared to octahedral sites; and X is present in two-third of them
a) So, ratio of X and Z equals 2×(23):1=4:3
⇒ formula of the compound = X4Z3
Assume a hypothetical cubic crystal lattice, named JEE-centered cubic (jcc) with the following characteristics:
I. An atom is present at all the corners of the cube
II. An atom is present at the center of two pairs of opposite faces
III. An atom is present at the center of all the edges of the cube
IV. One atom is present at its body-center
An element having the jcc lattice structure has a cell edge of 120 pm. The density of the element is 6.8 g/cm3. How many atoms are present in 408 g of the element?
:
C
Volume of unit cell =(120 pm)3=(120×10−12)3m3=(123×10−33)m3
Volume of 408 g of the element =massdensity=4086.8=60cm3=6×10−5m3
So, number of unit cells present in 408 g of the elements =6×10−5123×10−33=3.472×1025 unit cells
Since each jcc unit cell consist of 7 atoms,
therefore the total number of atoms presents in 408 g of the given element
=7×3.472×1025=2.43×1026atoms
Question 11-12 is based on the following given information:
Assume a hypothetical cubic crystal lattice, named JEE-centered cubic (jcc) with the following characteristics:
I. An atom is present at all the corners of the cube
II. An atom is present at the center of two pairs of opposite faces
III. An atom is present at the center of all the edges of the cube
IV. One atom is present at its body-center
How many atoms are effectively present per unit cell in this hypothetical crystal lattice?
:
B
I. 8 corner atom ×(18) atom per unit cell = 1 atom
II. 4 face-centered atoms ×(12) atom per unit cell = 2 atom
III. 12 edge-centered atoms ×(14) atom per unit cell = 3 atom
IV. 1 body centered atom × 1 atom per unit cell = 1 atom
So, Total number of atoms per unit cell =1+2+3+1=7 atoms