11th And 12th > Chemistry
P-BLOCK GROUP 18 - NOBLE GASES MCQs
Total Questions : 15
| Page 1 of 2 pages
Answer: Option B. ->
Helium atoms are small and cannot be retained by the Earth's gravitational field.
:
B
:
B
Helium accounts for up to 23 percent by mass of the Universe and the Sun. It is the most abundant element in the universe after hydrogen; Helium is rare in the atmosphere because its atoms are small and travel fast enough to escape the pull of Earth’s gravitational field. Do note that all the other noble gases occur in the atmosphere. There is no primordial Helium. In other words, there was no Helium from before the formation of Earth.
That leaves us with the question – where does the Helium on Earth come from? Helium comes into existence as α particles from radioactive decay and tends to accumulate in natural gas deposits.
Answer: Option B. ->
False
:
B
Xenon Fluorides are stored in Nickel or Monel containers. They cannot be stored in glass containers or quartz containers as they react with SiO2 as:
2XeF6(s)+SiO2(s)→SiF4(s)+2XeOF4(s)
2XeOF4(s)+SiO2(s)→SiF4(s)+2XeO2F2(s)
2XeO2F2(s)+SiO2(s)→SiF4(s)+2XeO3(s)
Also, the first two reactions may be used to prepare Xenon oxyfluorides. Further, Xenon oxyfluorides can also be prepared by the controlled, partial hydrolysis of Xenon fluorides.
Hydrolysis of XeF6 is a multi-step process. Initially Xenon oxide tetrafluoride – XeOF4 is formed.
XeF6(s)+H2O(l)→XeOF4(l)+2HF(l)
Followed by
XeOF4(l)+2H2O(l)→XeO3(s)+4HF(l)
:
B
Xenon Fluorides are stored in Nickel or Monel containers. They cannot be stored in glass containers or quartz containers as they react with SiO2 as:
2XeF6(s)+SiO2(s)→SiF4(s)+2XeOF4(s)
2XeOF4(s)+SiO2(s)→SiF4(s)+2XeO2F2(s)
2XeO2F2(s)+SiO2(s)→SiF4(s)+2XeO3(s)
Also, the first two reactions may be used to prepare Xenon oxyfluorides. Further, Xenon oxyfluorides can also be prepared by the controlled, partial hydrolysis of Xenon fluorides.
Hydrolysis of XeF6 is a multi-step process. Initially Xenon oxide tetrafluoride – XeOF4 is formed.
XeF6(s)+H2O(l)→XeOF4(l)+2HF(l)
Followed by
XeOF4(l)+2H2O(l)→XeO3(s)+4HF(l)
Answer: Option B. ->
XeOF4
:
A, C, and D
We had earlier seen that XeF2 has a linear shape. The 3 lone pairs of electrons of central Xe atom lie on the plane perpendicular to the axial Xe – F bonds. The molecule has a trigonal bipyramidal geometry
In this molecule, if one oxygen atom takes the place of a lone pair, we get the geometry of XeOF2. Do note that Xenon has a double bond with the oxygen atom. Let us look at the ground state and excited state:
Let us look at the geometry:
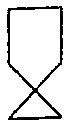
Now try to deduce the shape and geometry of XeO2F2:
A logical leap is to assume that the structure would be similar to replacing two of the three lone pairs with two oxygen atoms in the XeF2 structure. It is a good guess!
As for the geometry, XeF2, XeOF2 and XeO2F2 all have the same trigonal bipyramidal geometry. XeF2 has a linear shape. XeOF2 is T - Shaped. XeO2F2 is see-saw shaped.
Earlier we had seen that the geometry of XeF4 has a square bipyramidal geometry and square planar shape:

In the above geometry, if we replace a lone pair with an Oxygen atom such that there is a double bond between Xe and O, then we get the geometry of XeOF4. The four F atoms line in a plane perpendicular to the Xe – O axial sigma bond. This geometry is still the same as XeF4 but the shape will be square pyramidal!

So correct options are a c and d
:
A, C, and D
We had earlier seen that XeF2 has a linear shape. The 3 lone pairs of electrons of central Xe atom lie on the plane perpendicular to the axial Xe – F bonds. The molecule has a trigonal bipyramidal geometry

In this molecule, if one oxygen atom takes the place of a lone pair, we get the geometry of XeOF2. Do note that Xenon has a double bond with the oxygen atom. Let us look at the ground state and excited state:

Let us look at the geometry:

Now try to deduce the shape and geometry of XeO2F2:
A logical leap is to assume that the structure would be similar to replacing two of the three lone pairs with two oxygen atoms in the XeF2 structure. It is a good guess!

As for the geometry, XeF2, XeOF2 and XeO2F2 all have the same trigonal bipyramidal geometry. XeF2 has a linear shape. XeOF2 is T - Shaped. XeO2F2 is see-saw shaped.
Earlier we had seen that the geometry of XeF4 has a square bipyramidal geometry and square planar shape:

In the above geometry, if we replace a lone pair with an Oxygen atom such that there is a double bond between Xe and O, then we get the geometry of XeOF4. The four F atoms line in a plane perpendicular to the Xe – O axial sigma bond. This geometry is still the same as XeF4 but the shape will be square pyramidal!

So correct options are a c and d
Answer: Option A. ->
XeF6
:
A and B
Let us recall the hydrolysis reactions can we?
2XeF2(s)+2H2O(l)→2Xe(g)+O2(g)+4HF(l)
The above reaction easily disqualifies option c and d.
6XeF4(s)+12H2O(l)→4Xe(g)+3O2(g)+2XeO3(s)+24HF(l)
XeF6(S)+3H2O(l)→XeO3(s)+6HF(l)
Hydrolysis of XeF6 is a multi-step process. Initially Xenon oxide tetrafluoride – XeOF4 is formed.
XeF6(s)+H2O(l)→XeOF4(l)+2HF(l)
Followed by
XeOF4(l)+2H2O(l)→XeO3(s)+4HF(l)
:
A and B
Let us recall the hydrolysis reactions can we?
2XeF2(s)+2H2O(l)→2Xe(g)+O2(g)+4HF(l)
The above reaction easily disqualifies option c and d.
6XeF4(s)+12H2O(l)→4Xe(g)+3O2(g)+2XeO3(s)+24HF(l)
XeF6(S)+3H2O(l)→XeO3(s)+6HF(l)
Hydrolysis of XeF6 is a multi-step process. Initially Xenon oxide tetrafluoride – XeOF4 is formed.
XeF6(s)+H2O(l)→XeOF4(l)+2HF(l)
Followed by
XeOF4(l)+2H2O(l)→XeO3(s)+4HF(l)
Answer: Option A. ->
XeOF2
:
A, B, and D
XeOF2,XeOF4 and XeO2F2 are all well-known oxyfluorides of Xenon.
:
A, B, and D
XeOF2,XeOF4 and XeO2F2 are all well-known oxyfluorides of Xenon.
Answer: Option A. ->
XeOF2
:
Let us draw the Lewis structure for Xenon (VI) oxide or Xenon trioxide:
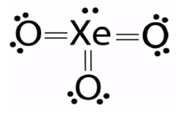
As we can see from the Lewis structure, there is one lone pair on the central atom and there are three atoms attached to the Xenon atom. Using VSEPR theory for a steric number = 3 bp + 1 lp = 4, we get a tetrahedral geometry and a pyramidal shape.
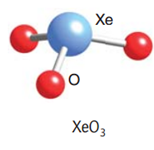
:
Let us draw the Lewis structure for Xenon (VI) oxide or Xenon trioxide:
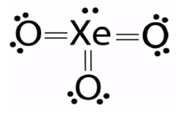
As we can see from the Lewis structure, there is one lone pair on the central atom and there are three atoms attached to the Xenon atom. Using VSEPR theory for a steric number = 3 bp + 1 lp = 4, we get a tetrahedral geometry and a pyramidal shape.

Answer: Option D. ->
No reaction
:
D
For the reaction,
Xe(g)+O2(g)→xenon oxidesΔfG∘ > 0
In other words, the standard change in free energy of the reaction is positive. Hence, this reaction is non-spontaneous. None of the Xenon oxides can be synthesized from this approach. So how can they be obtained?
:
D
For the reaction,
Xe(g)+O2(g)→xenon oxidesΔfG∘ > 0
In other words, the standard change in free energy of the reaction is positive. Hence, this reaction is non-spontaneous. None of the Xenon oxides can be synthesized from this approach. So how can they be obtained?
Answer: Option D. ->
No reaction
:
Xenon forms three fluorides:
Xe (g)+F2(g)400∘C 1atm−−−−−−−→XeF2(s) {Xe in excess}
Xe (g)+2F2(g)600∘C 6atm−−−−−−−→XeF4(s) {Xe:F2=1:1.5}
Xe (g)+3F2(g)300∘C 50atm−−−−−−−−→XeF6(s) {Xe:F2=1:20}
:
Xenon forms three fluorides:
Xe (g)+F2(g)400∘C 1atm−−−−−−−→XeF2(s) {Xe in excess}
Xe (g)+2F2(g)600∘C 6atm−−−−−−−→XeF4(s) {Xe:F2=1:1.5}
Xe (g)+3F2(g)300∘C 50atm−−−−−−−−→XeF6(s) {Xe:F2=1:20}
Answer: Option B. ->
False
:
B
Xenon Fluorides are clean fluorinating agents because usually they yield the fluorinated product and Xe gas. The required product can then easily be separated from Xe gas. In the following reaction,
XeF2 (s)+H2C=CH2(g)→Xe (g)+H2CF−FCH2 (g)
XeF2 is reduced to Xe (g). Definitely Xenon fluorides are not reducing agents. On the contrary, they behave as oxidizing agents. For example,
XeF4 (s)+2SF4 (g)→Xe (g)+2SF6 (g)
:
B
Xenon Fluorides are clean fluorinating agents because usually they yield the fluorinated product and Xe gas. The required product can then easily be separated from Xe gas. In the following reaction,
XeF2 (s)+H2C=CH2(g)→Xe (g)+H2CF−FCH2 (g)
XeF2 is reduced to Xe (g). Definitely Xenon fluorides are not reducing agents. On the contrary, they behave as oxidizing agents. For example,
XeF4 (s)+2SF4 (g)→Xe (g)+2SF6 (g)
Answer: Option A. ->
True
:
A
True.
2XeF2(s)+2H2O(l)→2Xe (g)+O2 (g)+4HF(l)
6XeF4 (s)+12H2O (l)→4Xe (g)+3O2 (g)+2XeO3 (s)+24HF (l)
XeF6(s)+3H2O (l)→XeO3 (s)+6HF (l)
Hydrolysis of XeF6 is a multi-step process. Initially Xenon oxide tetrafluoride – XeF4 is formed.
XeF6(s)+H2O (l)→XeOF4 (l)+2HF (l)
Followed by
XeOF4 (l)+2H2O (l)→XeO3 (s)+4HF (l)
:
A
True.
2XeF2(s)+2H2O(l)→2Xe (g)+O2 (g)+4HF(l)
6XeF4 (s)+12H2O (l)→4Xe (g)+3O2 (g)+2XeO3 (s)+24HF (l)
XeF6(s)+3H2O (l)→XeO3 (s)+6HF (l)
Hydrolysis of XeF6 is a multi-step process. Initially Xenon oxide tetrafluoride – XeF4 is formed.
XeF6(s)+H2O (l)→XeOF4 (l)+2HF (l)
Followed by
XeOF4 (l)+2H2O (l)→XeO3 (s)+4HF (l)
















