11th And 12th > Chemistry
P-BLOCK GROUP 16 - CHALCOGENS MCQs
Total Questions : 15
| Page 1 of 2 pages
Answer: Option C. ->
H2O
:
C
As we go down the group, the electronegativity of the central atom decreases while the atomic size increases. This causes the E−H bond to weaken as we go down the group. Hence the thermal stability order is as H2O>H2S>H2Se>H2Te>H2Po
:
C
As we go down the group, the electronegativity of the central atom decreases while the atomic size increases. This causes the E−H bond to weaken as we go down the group. Hence the thermal stability order is as H2O>H2S>H2Se>H2Te>H2Po
Answer: Option C. ->
H2O
:
Hydrogen peroxide readily breaks down in the presence of finely divided metals to liberate oxygen gas:
2H2O2+Metal→2H2O(l)+O2(g)
:
Hydrogen peroxide readily breaks down in the presence of finely divided metals to liberate oxygen gas:
2H2O2+Metal→2H2O(l)+O2(g)
Answer: Option C. ->
Both (A) and (B)
:
C
Among the Chalcogens, Oxygen shows considerable anomalies. For instance, Oxygen exists as a diatomic gas while the other chalcogens are all solids at room temperature and 1atm pressure. Further, oxygen forms strong and stable pπ−pπ double bonds (as in the case of O2 molecule) whereas the other elements tend not to. Also, the Oxygen - Oxygen single bond is weaker compared to the Element - Element single bonds of other chalcogens.
All this can be attributed to the small atomic size and high electro negativity of Oxygen.
:
C
Among the Chalcogens, Oxygen shows considerable anomalies. For instance, Oxygen exists as a diatomic gas while the other chalcogens are all solids at room temperature and 1atm pressure. Further, oxygen forms strong and stable pπ−pπ double bonds (as in the case of O2 molecule) whereas the other elements tend not to. Also, the Oxygen - Oxygen single bond is weaker compared to the Element - Element single bonds of other chalcogens.
All this can be attributed to the small atomic size and high electro negativity of Oxygen.
Answer: Option D. ->
All of these
:
D
Strongly heating Potassium dichromate, Potassium Permanganate or Potassium Chlorate gives oxygen gas.
Heating above 400∘C, the following reactions takes place
4K2Cr2O7→4K2Cr2O4+2Cr2O3+3O2
2KMnO4→K2MnO4+MnO2+O2
2KClO3→2KCl+3O2
Thus all of them release oxygen gas on heating above 400∘C. These reactions can be used in the preparation of oxygen gas in laboratory.
:
D
Strongly heating Potassium dichromate, Potassium Permanganate or Potassium Chlorate gives oxygen gas.
Heating above 400∘C, the following reactions takes place
4K2Cr2O7→4K2Cr2O4+2Cr2O3+3O2
2KMnO4→K2MnO4+MnO2+O2
2KClO3→2KCl+3O2
Thus all of them release oxygen gas on heating above 400∘C. These reactions can be used in the preparation of oxygen gas in laboratory.
Answer: Option A. ->
True
:
A
In an electrolytic setup anode is the place where oxidation takes place and cathode is the place where reduction takes place. O−2 looses two electrons at the anode to form oxygen, where as H+ gain electron at the cathode to form hydrogen gas.
Oxidation is nothing but loss of electrons and reduction is nothing but gain of electrons.
Electrolysis of water gives oxygen (O2) gas at anode and hydrogen (H2) gas at cathode and it can be separated and stored in cylinders
:
A
In an electrolytic setup anode is the place where oxidation takes place and cathode is the place where reduction takes place. O−2 looses two electrons at the anode to form oxygen, where as H+ gain electron at the cathode to form hydrogen gas.
Oxidation is nothing but loss of electrons and reduction is nothing but gain of electrons.
Electrolysis of water gives oxygen (O2) gas at anode and hydrogen (H2) gas at cathode and it can be separated and stored in cylinders
Answer: Option D. ->
Inspiration and combustion
:
D
Oxygen is the major constituent during inspiration (inhalation) and combustion because both these processes cannot occur without oxygen. Oxygen from inspiration is used to combust the food to release energy and any fuel when combusted, releases energy. Hence oxygen is a must for combustion.
:
D
Oxygen is the major constituent during inspiration (inhalation) and combustion because both these processes cannot occur without oxygen. Oxygen from inspiration is used to combust the food to release energy and any fuel when combusted, releases energy. Hence oxygen is a must for combustion.
Answer: Option A. ->
True
:
A
If the oxygen gas is not Humidified before entering patient’s body, it then causes drying of upper respiratory tract. Therefore, always humidified oxygen is used in hospitals to supply to the patients.
:
A
If the oxygen gas is not Humidified before entering patient’s body, it then causes drying of upper respiratory tract. Therefore, always humidified oxygen is used in hospitals to supply to the patients.
Question 8.
We humans have known elemental Sulphur from the earliest times. In the last two decades, the allotropy of sulfur has been explored in great detail. The most common naturally occurring allotrope,S8 cyclo-octasulphur, has a puckered three dimensional “crown” like arrangement of atoms around the ring. This homocyclic allotrope forms “needle-like” crystals above 95∘ C, but below that temperature, crystallizes in a “chunky” fashion. The crystals, which are referred to as monoclinic and rhombic forms, differ simply in the way in which the molecules pack. These are allotropes of each other – True or False?
Answer: Option B. ->
False
:
B
The question looks long for a true or false type doesn’t it. Shall we keep the answer very short – yes?
Polymorphs are defined as different crystal forms in which identical units of the same compound are packed differently. Strictly speaking, the three varieties of cyclo-octasulphur – the α -Sulphur, β -Sulphur and the γ -Sulphur are all polymorphs and not allotropes. Allotropes contain different molecular units!
The repeating molecular unit of cyclo-octasulphur – the S8 molecule looks like:
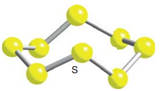
:
B
The question looks long for a true or false type doesn’t it. Shall we keep the answer very short – yes?
Polymorphs are defined as different crystal forms in which identical units of the same compound are packed differently. Strictly speaking, the three varieties of cyclo-octasulphur – the α -Sulphur, β -Sulphur and the γ -Sulphur are all polymorphs and not allotropes. Allotropes contain different molecular units!
The repeating molecular unit of cyclo-octasulphur – the S8 molecule looks like:

Answer: Option A. ->
True
:
A
When heated to 113∘C Orthorhombic sulphur melts and if we continue heating this molten yellow liquid, it darkens above 160∘C and becomes more viscous as the Sulphur rings break open and polymerize. The resulting helical Sn polymers can be extracted from the melt by pouring the molten Sulphur in water (called quenching). This super-cooled liquid is a metastable rubber-like material that slowly converts back to stable α−S8 at room temperature. Different polymeric chains can have different number of atoms and isolating a particular strain becomes difficult. Obviously these polymeric forms are amorphous
:
A
When heated to 113∘C Orthorhombic sulphur melts and if we continue heating this molten yellow liquid, it darkens above 160∘C and becomes more viscous as the Sulphur rings break open and polymerize. The resulting helical Sn polymers can be extracted from the melt by pouring the molten Sulphur in water (called quenching). This super-cooled liquid is a metastable rubber-like material that slowly converts back to stable α−S8 at room temperature. Different polymeric chains can have different number of atoms and isolating a particular strain becomes difficult. Obviously these polymeric forms are amorphous
Answer: Option B. ->
Sulphur
:
B
Among the chalcogens, Sulphur exhibits the greatest tendency for catenation owing to the S−S bond stability.
:
B
Among the chalcogens, Sulphur exhibits the greatest tendency for catenation owing to the S−S bond stability.
















