MCQs
Heat Radiation, Thermal Properties Of Matter, Dual Nature Of Matter
Total Questions : 247
| Page 2 of 25 pages
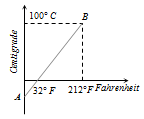
Answer: Option B. -> Slope of line AB is 59
:
B
Relation between Celsius and Fahrenheit scale of temperature is C5=F−329
By rearranging we get, C = 59F−1609
By equating above equation with standard equation of line y=mx+c we get m=59 and c=−1609
i.e. Slope of the line AB is 59.
:
B
Relation between Celsius and Fahrenheit scale of temperature is C5=F−329
By rearranging we get, C = 59F−1609
By equating above equation with standard equation of line y=mx+c we get m=59 and c=−1609
i.e. Slope of the line AB is 59.
Question 12. In a constant volume gas thermometer, the pressure of the working gas is measured by the difference in the levels of mercury in the two arms of a U-tube connected to the gas at one end. When the bulb is placed at the room temperature 27.0℃, the mercury column in the arm open to atmosphere stands 5.00 cms above the level of mercury in the other arm. When the bulb is placed in a hot liquid, the difference of mercury levels becomes 45.0 cms. Calculate the temperature of the liquid. (Atmospheric pressure = 75.0 cm of mercury.)
Answer: Option B. -> 177℃
:
B
The pressure of the gas = atmospheric pressure + the pressure due to the difference in mercury levels
At 27℃, the pressure is (75 cm + 5 cm) = 80 cm of mercury. At the liquid's temperature, the pressure is (75 cm + 45 cm) = 120 cm of mercury
Using T2=(P2P1)T1,the temperature of the liquid is -
T=[(12080)×(27.0+273.15)]K=450.22K=177.07∘C≈177∘C.
:
B
The pressure of the gas = atmospheric pressure + the pressure due to the difference in mercury levels
At 27℃, the pressure is (75 cm + 5 cm) = 80 cm of mercury. At the liquid's temperature, the pressure is (75 cm + 45 cm) = 120 cm of mercury
Using T2=(P2P1)T1,the temperature of the liquid is -
T=[(12080)×(27.0+273.15)]K=450.22K=177.07∘C≈177∘C.
Question 13. The ideal gas law, which relates the P, V and temperature T of an ideal gas in a closed box, given as PV = nRT, can be manipulated to construct an effective thermometer. A "constant volume gas thermometer” uses a sample of gas (commonly, nitrogen or helium) in a closed chamber, thus fixing the volume V. The temperature is then measured by reading off the corresponding pressure P. At the freezing point of water, it is seen that a gas thermometer records a pressure of 0.9 x 105 Pa; it also records a pressure of 1.2 x 105 Pa at the boiling point of water. What pressure will you find in the gas chamber at a room temperature of 230C?
Answer: Option D. -> 0.97 x 105 Pa.
:
D
For an ideal gas at constant V,
P∝ T
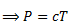 (for some proportionality constant 'C').
(for some proportionality constant 'C').
Let (TF, PF) and (TB,PB) be the (T,P) values for the freezing and the boiling points of water respectively. We can write -
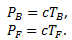 (1)
(1)
Subtracting,
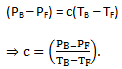 (2)
(2)
From relation (1) and (2), we can write -
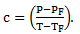 (3)
(3)
Comparing (2) and (3),
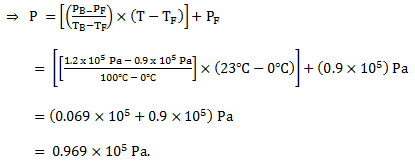
:
D
For an ideal gas at constant V,
P∝ T
Let (TF, PF) and (TB,PB) be the (T,P) values for the freezing and the boiling points of water respectively. We can write -
Subtracting,
From relation (1) and (2), we can write -
Comparing (2) and (3),
Question 14. Two rods, one of aluminium and the other made of steel, having initial length l1 and l2 are connected together to form a single rod of length l1+l2. The coefficients of linear expansion for aluminium and steel are αa and αa and respectively. If the length of each rod increases by the same amount when their temperature are raised by toC, then find the ratio of length of aluminium rod to the whold rod.
(IIT JEE 2003)
(IIT JEE 2003)
Answer: Option C. -> αs/(αa+αs)
:
C
The length of each rod increases by the same amount
∴△la=△ls⇒△I1αat=l2αst
⇒l2l1=αaαs⇒l2l1+1=αaαs+1
⇒l2+l1l1=αa+αsαs⇒l1l1+l2=αsαa+αs
:
C
The length of each rod increases by the same amount
∴△la=△ls⇒△I1αat=l2αst
⇒l2l1=αaαs⇒l2l1+1=αaαs+1
⇒l2+l1l1=αa+αsαs⇒l1l1+l2=αsαa+αs
Question 15. In a closed container of constant volume, the pressure of water exhibits an interesting dependence on temperature -
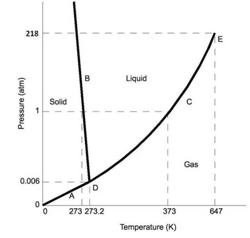
The point D (273.16 K,0.006 atm) is called the triple point, Tp, of water, where all there phases coexist. What is the observed phase change when the temperature is increased from -100C to +100C, while maintaining a constant pressure of 0.006 atm?
The point D (273.16 K,0.006 atm) is called the triple point, Tp, of water, where all there phases coexist. What is the observed phase change when the temperature is increased from -100C to +100C, while maintaining a constant pressure of 0.006 atm?
Answer: Option C. -> Solid → Gas
:
C
Along the constant pressure line of P=0.006, we have ice (solid H2O) on the left of the triple point, and steam (gaseous H2O) on the right.
Thus, as we increase the temperature from below Tpto above Tp, we observe a solid → gas phase transition, i.e., we see ice directly turning into gas without going through the water phase. Magical!
:
C
Along the constant pressure line of P=0.006, we have ice (solid H2O) on the left of the triple point, and steam (gaseous H2O) on the right.
Thus, as we increase the temperature from below Tpto above Tp, we observe a solid → gas phase transition, i.e., we see ice directly turning into gas without going through the water phase. Magical!
Answer: Option C. -> α- particle
:
C
KE=12mv2=p22mp=√2mE∴λ=h√2mE⇒λα1√mmp=1836memα=4×1836methereforemα>mp>me
⇒λα is the shortest
:
C
KE=12mv2=p22mp=√2mE∴λ=h√2mE⇒λα1√mmp=1836memα=4×1836methereforemα>mp>me
⇒λα is the shortest
Answer: Option A. -> λ
:
A
λ=hmv and E=12mv2⇒λ=hv2E when v and E both are doubled, λ remains unchanged i.e.λ′=λ.
:
A
λ=hmv and E=12mv2⇒λ=hv2E when v and E both are doubled, λ remains unchanged i.e.λ′=λ.
Answer: Option A. -> 1:1
:
A
λ=hmvλd=h2mp×2v=h4×mpv...(1)λα=h4mp×v=h4×mpv...(2)
From (1) and (2)
λd:λα=1:1
:
A
λ=hmvλd=h2mp×2v=h4×mpv...(1)λα=h4mp×v=h4×mpv...(2)
From (1) and (2)
λd:λα=1:1
Answer: Option B. -> 1.6×10−21
:
B
p=EC=3×106×1.6×10−193×108=1.6×10−21kgms−1
:
B
p=EC=3×106×1.6×10−193×108=1.6×10−21kgms−1
Answer: Option B. -> 1.4×103
:
B
If momenta of the electron and photon are equal, their wavelenghths should also be equal.
λ=hmv⇒v=hmλ=6.6×10−349.1×10−31×5200×10−10⇒v=1.4×103m/s.
:
B
If momenta of the electron and photon are equal, their wavelenghths should also be equal.
λ=hmv⇒v=hmλ=6.6×10−349.1×10−31×5200×10−10⇒v=1.4×103m/s.