Question
The ideal gas law, which relates the P, V and temperature T of an ideal gas in a closed box, given as PV = nRT, can be manipulated to construct an effective thermometer. A "constant volume gas thermometer” uses a sample of gas (commonly, nitrogen or helium) in a closed chamber, thus fixing the volume V. The temperature is then measured by reading off the corresponding pressure P. At the freezing point of water, it is seen that a gas thermometer records a pressure of 0.9 x 105 Pa; it also records a pressure of 1.2 x 105 Pa at the boiling point of water. What pressure will you find in the gas chamber at a room temperature of 230C?
Answer: Option D
:
D
For an ideal gas at constant V,
P∝ T
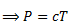 (for some proportionality constant 'C').
(for some proportionality constant 'C').
Let (TF, PF) and (TB,PB) be the (T,P) values for the freezing and the boiling points of water respectively. We can write -
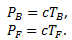 (1)
(1)
Subtracting,
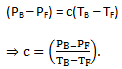 (2)
(2)
From relation (1) and (2), we can write -
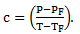 (3)
(3)
Comparing (2) and (3),
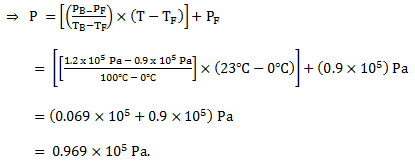
Was this answer helpful ?
:
D
For an ideal gas at constant V,
P∝ T
Let (TF, PF) and (TB,PB) be the (T,P) values for the freezing and the boiling points of water respectively. We can write -
Subtracting,
From relation (1) and (2), we can write -
Comparing (2) and (3),
Was this answer helpful ?


















Submit Solution