11th And 12th > Chemistry
GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS MCQs
:
D
Electromagnetic separation is applicable when either the ore particles or the impurities are magnetic (attracted by a magnet) in nature. Electromagnets present in a rotor attracts magnetic particles forming a separate heap from non-magnetic particles.
:
A
Gravity separation also known as hydraulic washing is based on the differences in weight between the gangue and the metal. In general, the metal particles of the ore are heavier than the impurities. So we use the principle of gravity separation to separate the two.
Finely pulverized (powdered) ore is treated with a stream of water such that the lighter gangue particles are washed away leaving behind the heavier ore particles. This is particularly applicable for heavier ore like Cassiterite and Haematite.
:
B, C, and D
During the production of iron, the oxide ore is predominantly reduced to iron by CO (carbon monoxide) and not by coke. In the reduction zone, the reduction takes place between 250 0C– 700 0C. In this temperature range, CO is a better reducing agent than carbon. The reaction takes place as follows:
Fe2O3(s)+3CO(g)→2Fe(l)+3CO2(g)
The silica impurities formed during the production of iron are removed as calcium silicate slag on reaction with calcium oxide. The reaction takes place as follows:
CaO(s)+SiO2(s)→CaSiO3(s) (Slag)
This silicate slag is used in the manufacturing of cement.
:
A
FeO (Basic gangue) impurity is mixed with SiO2 (acidic flux) to form a fusible slag
FeO +SiO2 → FeSiO3
(slag)
:
C and D
The roasted ore is mixed with Silica and powdered coke (to deter the oxidation of FeO to Fe2O3 so that FeO could be removed as FeSiO3)
FeO + SiO2 → FeSiO3
The temperature is high enough to melt the slag. Below the layer of slag is molten mass known as Copper Matte. It consists of Cu2S and FeS
:
B
Red Bauxite contains Al2O3.2H2O +Fe2O3 (major impurity) + SiO2 +TiO2
After calcination, organic matter, volatile substances and moisture are removed. The calcine is then leached with 45 % NaOH solution at about 150∘C where
Note that only TiO2 and Fe2O3 are filtered out.
:
C
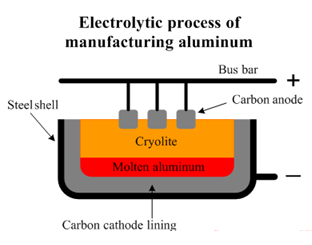
In the electrolytic extraction of Aluminium, purified alumina is mixed with Na3AlF6 and CaF2. Cryolite (Na3AlF6) helps dissolve Alumina and lowers the melting point of this mix to about 1050 ∘C. Pure Alumina melts at about 2100 ∘C. CaF2 is added to enhance the conductivity.
As shown in the above image, the surface on the inside of the steel case is coated with a graphite lining, which acts as the cathode. The electrolyte is a molten mixture of cryolite, molten Alumina and CaF2.
:
A spontaneous process is one that needs no intervention from outside. It is also called a irreversible reaction. It releases free energy and moves to a lower, thermodynamically more stable state. The change in free energy is negative.
:
C
van Arkel Method: The impure metal (Zr and Ti) are converted to a volatile compound, impurities being unaffected. The formed pure volatile compound is decomposed to get the pure metal. Hence this is a method to obtain ultra pure metal by refining. Hf & Si can also be purified by van Arkel method.
:
A
Let us look at the chief ores of the given metals:
Copper: CuFeS2 (chalcopyrites).
Silver: Ag2S (Argentite)
Lead: PbS (Galena)