11th And 12th > Chemistry
GENERAL ORGANIC CHEMISTRY MCQs
:
B
A free-radical reaction is any chemical reaction involving free radicals.
The major product is based on the most stable free radical.
These reactions usually involve 3 major steps: chain initiation, chain propagation and chain termination.
These are usually initiated by U.V light or other light sources which have enough energy to initiate the reaction.
:
C
This is a good example of a photochemical reaction. A reaction brought about by light.
One of the hydrogen atoms in the methyl group has been replaced by a chlorine atom, so this is a substitution reaction. However, the reaction doesn't stop there, and all three hydrogens in the methyl group can, in turn, be replaced by chlorine atoms.
:
B
Look at the structure of benzene.
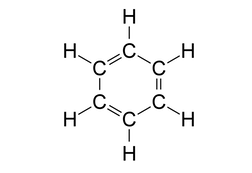
Every carbon atom in the benzene ring has a double bond with a neighbouring carbon atom and a single bond with another carbon atom. It is also bonded to one hydrogen atom with a single bond.
So, each carbon atom has 3 sigma bonds and 1 pi bond. This is possible only if the carbon atom is sp2 hybridized where we get 3 sp2 hybrid orbitals to form 3 sigma bonds and 1 unhybridised p orbital to form a pi bond.
Another feature of sp2 hybridisation is that it leads to a trigonal planar geometry which is also true in this case.
:
C
It so happens that the most common type of reaction in aromatic compounds is an electrophilic substitution reaction.
This is because the benzene molecule is electron-rich.
:
D
When a methyl group is attached to an alkene or conjugated system, it shows maximum hyperconjugation.
CH3−CH=CH2 → Has 3 hyper conjugative structures.
CH3CH2−CH=CH2 → Has 2 hyper conjugative structures.
+I effect of ethyl is more than methyl but methyl shows more hyperconjugation due to the presence of higher number of alpha hydrogens.

:
D
The starred carbon in d are not present on sp2 hybrid carbon
:
C
Carbon which has 3σ bonds and 1π bond in C-3 and C-5 sp2 hybridised.
Carbon which has 3σ bonds and 1π bond as in C-4 is sp hybridised.
:
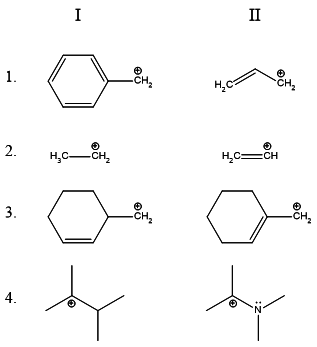
B
For part 1., we have to choose the more stable of benzyl and allyl
carbocations. The benzyl carbocation has more resonance contributors.
In theory, it can be considered to be more stable.
For part 2., we have to choose the more stable cation between an
1∘ alkyl and a vinyl cation. The latter is more unstable because
the positive charge rests on a carbon having a double bond - which has
more % s-character and is thus more electronegative than the alkane's
carbon bearing the positive charge. The primary alkyl carbocation is thus
more stable.
For part 3., we have a straightforward case where an allyl carbocation is
more stable than a primary alkyl carbocation.
For part 4., the lone pair on the adjacent nitrogen stabilizes the positive charge.
Hence the resonance stabilized carbocation is more stable!
:
B
C1H3 − C3H|C2H3 − C5H|C4H3 − C6H3
Carbons labelled 2 & 3 are tertiary carbons, it is carbon attached with 3 other carbon atoms and rest of the carbons are primary in nature.