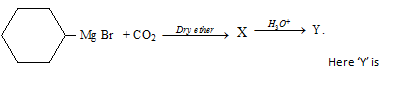11th And 12th > Chemistry
CARBOXYLIC ACIDS MCQs
Total Questions : 15
| Page 1 of 2 pages
Answer: Option B. ->
Terephthalic acid
:
B
:
B
It is oxidation and - COOH groups are in para position
Answer: Option C. ->

:
C

:
C
The type acid depends upon R group of Grignard
reagent
Answer: Option C. ->
A and P are same but C and Q are same
:
C
:
C
in oxidation of side chain only one carbon of aromatic ring is retained
Answer: Option D. ->
CH2 = CH - CH2OH
:
D
LiAlH4 cannot reduce double bond
:
D
LiAlH4 cannot reduce double bond
Answer: Option D. ->

:
D

:
D
side chain containing 30carbon(or) carbon with out hydrogen will not get oxidised
Answer: Option A. ->
Succinic acid
:
A
:
A
A is ethylene, B is vicdibromide , C is NC - CH2 - CH2 -
CN
Answer: Option D. ->
X = CH3COOC2H5, Y = CH3COOH, A = CH3COOK, B = C2H5OH
:
D
:
D
First reaction saponification of ester & Last reaction is neutralisation
Answer: Option D. ->
(a) and (b) above.
:
D
:
D
Both a & b plays role in more acidic nature of acids
Answer: Option A. ->
Dimer, Polymer
:
A
:
A
In vapour 2 molecules are found together due to inter molecular hydrogen bond & in liquid many molecules are found together due to inter molecular hydrogen bond