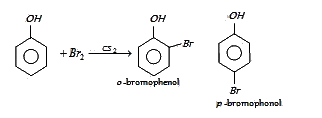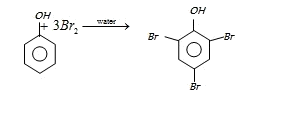11th And 12th > Chemistry
ALCOHOLS PHENOLS AND ETHERS MCQs

:
B
Reaction proceeds by benzyl carbocation formation which is stabilized due to resonance
:
B
In presence of non-polar solvent (CS2) the ionization of phenol is suppressed. The ring is slightly activated and hence mono substitution occurs with p-bromophenol as the major product.
On the other hand with water phenol forms 2, 4, 6-tribromo phenol. (CS2)
In aqueous solution phenol ionizes to give phenoxide ion. Due to the presence of negative charge of
oxygen the benzene ring is highly activated and hence trisubstituted product is obtained.
:
C
Strong oxidising agents like acidified KMnO4 or K2Cr2O7 oxidised alcohols to carboxylic acids. For oxidation of primary alcohols to ketones, Pyridinium chloro-chromate(PCC) is used and for oxidation of secondary alcohols to ketones, Chromic anhydride in glacial acetic acid is used.
:
C
Order of Acidity− o−nitrophenol> phenol> o−cresol> ethanol. o−nitrophenol is more acidic than phenol due to electron withdrawing nature of nitro group. o-crseol is less acidic than alcohol due to electron-releasing nature of methyl group. Phenoxide ion is resonance stabilized but ethoxide ion is not. Thus, phenol is more acidic than alcohol. Greater the pKavalue, less is the acidity.
:
D
Diethyl ether is C4H10O. n-propylmethylether, butan-1-ol & 2-methyl~propan-2-ol is C4H10O. Butanone is C4H8O.
Ortho-nitrophenol is steam volatile whereas para-nitrophenol is not. This is due to
1.Intramolecular hydrogen bonding present in ortho-nitrophenol
2.Intermolecular hydrogen bonding present in para-nitrophenol
3.Intramolecular hydrogen bonding present in para-nitrophenol
4.Inter-molecular hydrogen bonding present in ortho-nitrophenol.
:
C
Para-nitro phenol has higher boiling point than ortho-nitrophenol due to intermolecular hydrogen bonding present in para-nitrophenol, which requires more energy to break these bonds during boiling. In o-nitrophenol, intramolecular hydrogen bonding is present to greater extent. Thus, o-nitrophenol is steam volatile due to low boiling point.
:
C
When propene reacts with mercuric acetate followed by NaBH4, it gives secondary or tertiary alcohols, in accordance with Markownikoff’s rule. But, with diborane followed by H2O2, it gives primary alcohols which is in accordance with Anti-Markownikoff’s rule. 6CH3−CH=CH2+B2H6H2O2−−−→CH3−CH2−CH2OH
:
C
Friedel-Craft's acylation of anisole with acetyl chloride in presence of anhydrous AlCl3 gives 4-methoxy acetophenone(para) as a major product. Methoxy group in anisole is activating and ortho-para directing with major product being para isomer.
:
B
C2H5OH (ethanol) is a very weak acid hence it does not react with NaOH. However, it reacts with metallic sodium, giving sodium ethoxide and hydrogen gas.