Question
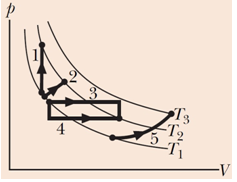
The figure here shows five paths traversed by a gas on a P-V diagram. Rank the paths according to the change in internal energy of the gas, greatest first.
Answer: Option D
:
D
For any ideal gas, the internal energy can be related to the temperature as -
Eint=nCvT
⇒ΔEint=nCvΔT (for a change of state).
Thus the change in internal energy is solely dictated by the change in temperature.Since, all the processes 1, 2, 3, and 4 begin and end at the same temperatures, their ΔT′s(and,ΔEint=nCv(T2−T1)) are the same. Process 5, on the other hand goes through a larger ΔT(T3−T1), hence gains the highest internal energy.
Was this answer helpful ?
:
D
For any ideal gas, the internal energy can be related to the temperature as -
Eint=nCvT
⇒ΔEint=nCvΔT (for a change of state).
Thus the change in internal energy is solely dictated by the change in temperature.Since, all the processes 1, 2, 3, and 4 begin and end at the same temperatures, their ΔT′s(and,ΔEint=nCv(T2−T1)) are the same. Process 5, on the other hand goes through a larger ΔT(T3−T1), hence gains the highest internal energy.
Was this answer helpful ?


















Submit Solution