Question
Figure below represents a Carnot engine that works between temperatures T1 400 K and T2 150 K and drives aCarnot refrigerator that works between temperatures T3 325 K and T4 225 K.What is the ratio Q3Q1?
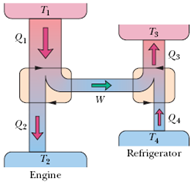
Answer: Option C
:
C
Let us consider the efficiency of the heat engine first.
ηH.E=1−T2T1
=1−150400=58
∴W=ηH.E×Q1
=58Q1.......................(1)
Now this work is utilized by the refrigerator to add heat to the hot body from the cold body. The efficiency of this refrigerator is calculated using.
ηr=1−T3T4
=1−225325
=413...........(2)
We know that ηr=Q2w
⇒Q2=w×ηr ............(3)
From 1st law of thermodynamics we know
Q3=Q2+w
=w+w×ηr - - - - - - (4)
Substituting for w and etar in (4) we get
Q3=58Q1+58Q1×413
=Q1(65+208×13)
=Q1×85104
Q3Q1=85104
Was this answer helpful ?
:
C
Let us consider the efficiency of the heat engine first.
ηH.E=1−T2T1
=1−150400=58
∴W=ηH.E×Q1
=58Q1.......................(1)
Now this work is utilized by the refrigerator to add heat to the hot body from the cold body. The efficiency of this refrigerator is calculated using.
ηr=1−T3T4
=1−225325
=413...........(2)
We know that ηr=Q2w
⇒Q2=w×ηr ............(3)
From 1st law of thermodynamics we know
Q3=Q2+w
=w+w×ηr - - - - - - (4)
Substituting for w and etar in (4) we get
Q3=58Q1+58Q1×413
=Q1(65+208×13)
=Q1×85104
Q3Q1=85104
Was this answer helpful ?


















Submit Solution