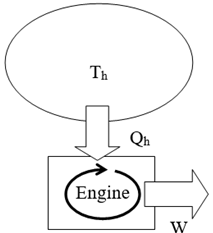
Question
In one complete cycle, a heat engine extracts heat Qh from a thermal reservoir, does work W and does not eject any heat into the environment. This heat engine is impossible because its operation violates:
Answer: Option B
:
B
This would violate the 2nd Law only. The entropy of the hot reservoir decreases by ΔS=QT. The entropy of the engine did not change (since it returns to its initial state). The entropy of the rest of the universe did not change, since doing pure work does not change entropy.
Was this answer helpful ?
:
B
This would violate the 2nd Law only. The entropy of the hot reservoir decreases by ΔS=QT. The entropy of the engine did not change (since it returns to its initial state). The entropy of the rest of the universe did not change, since doing pure work does not change entropy.
Was this answer helpful ?


















Submit Solution