Question
In the commercial electrochemical process for Aluminium extraction, the electrolyte used is:
Answer: Option C
:
C
In the electrolytic extraction of Aluminium, purified alumina is mixed with Na3AlF6 and CaF2. Cryolite (Na3AlF6) helps dissolve Alumina and lowers the melting point of this mix to about 1050 ∘C. Pure Alumina melts at about 2100 ∘C. CaF2 is added to enhance the conductivity.
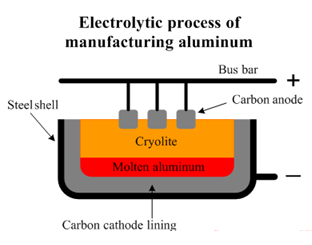
As shown in the above image, the surface on the inside of the steel case is coated with a graphite lining, which acts as the cathode. The electrolyte is a molten mixture of cryolite, molten Alumina and CaF2.
Was this answer helpful ?
:
C
In the electrolytic extraction of Aluminium, purified alumina is mixed with Na3AlF6 and CaF2. Cryolite (Na3AlF6) helps dissolve Alumina and lowers the melting point of this mix to about 1050 ∘C. Pure Alumina melts at about 2100 ∘C. CaF2 is added to enhance the conductivity.

As shown in the above image, the surface on the inside of the steel case is coated with a graphite lining, which acts as the cathode. The electrolyte is a molten mixture of cryolite, molten Alumina and CaF2.
Was this answer helpful ?


















Submit Solution