Question
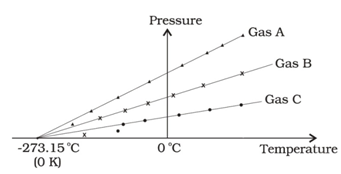
Given that P, V and T stand for vapour pressure, volume and temperature, an ideal gas obeys the law -PV α T. If the volume is kept constant, P α T. With decreasing temperature the vapour pressure drops, until P = 0 at some temperature T0. Since P cannot be less than zero, or negative, T0 naturally becomes the lowest temperature that we can reach, and can be used as a "natural” lower fixed point while constructing a temperature scale. This is the "absolute zero”, which in the Celsius scale is - 273.150C.
To construct an absolute temperature scale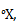 , where the absolute zero is naturally 0
, where the absolute zero is naturally 0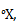 . Let us choose the triple point of mercury (-38.80C, at 0.2 mPa) as our upper fixed point, and give assign it a value 100
. Let us choose the triple point of mercury (-38.80C, at 0.2 mPa) as our upper fixed point, and give assign it a value 100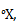 . What will be the boiling point of water on this scale?
. What will be the boiling point of water on this scale?
To construct an absolute temperature scale


















Submit Solution