Question
Answer: Option B
:
B
In the given projection formula, there is only one stereogenic center.
It is the carbon attached to the −COOH group! Apart from the carboxylicacid group, there is an −NH2 group, a −H and a CSH(Me)_2. Since there are four different groups on the carbon, it is a chiral centre.
The carbon attached to two methyl groups is clearly not chiral.
Let us convert the projection into a wedge-dash format and take itfrom there:
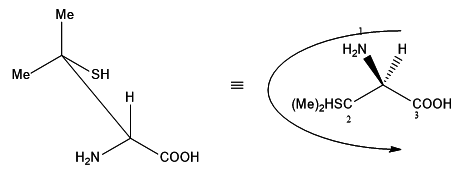
As we know hydrogen has the least atomic number, 'H' takes number '4' and in the wedge-dash notation shown above, hydrogen is below the plane. The carbon attached to the sulphur(SCC) takes number '2'. It has higher precedence than the carboxylic acidcarbon (OOO) which will take number '3'. Now we can see that the numbers are making an arc in the anti-clockwise direction. Hence given compound is in S configuration.
Was this answer helpful ?
:
B
In the given projection formula, there is only one stereogenic center.
It is the carbon attached to the −COOH group! Apart from the carboxylicacid group, there is an −NH2 group, a −H and a CSH(Me)_2. Since there are four different groups on the carbon, it is a chiral centre.
The carbon attached to two methyl groups is clearly not chiral.
Let us convert the projection into a wedge-dash format and take itfrom there:

As we know hydrogen has the least atomic number, 'H' takes number '4' and in the wedge-dash notation shown above, hydrogen is below the plane. The carbon attached to the sulphur(SCC) takes number '2'. It has higher precedence than the carboxylic acidcarbon (OOO) which will take number '3'. Now we can see that the numbers are making an arc in the anti-clockwise direction. Hence given compound is in S configuration.
Was this answer helpful ?
More Questions on This Topic :
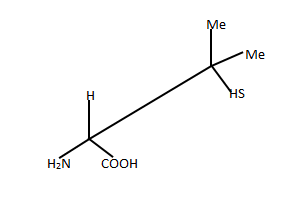
Question 3. What about this compound? Is it chiral?....


















Submit Solution