Question
Answer: Option A
:
A
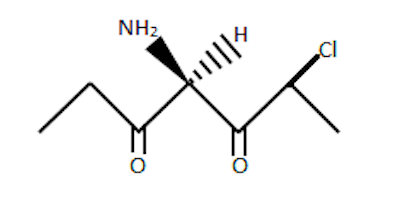
Look at the carbon where stereochemical data is given.
Now, we need to figure out if there are any elements of symmetry.
If we draw a plane that passes through this carbonand −H and −NH2,
we don't get two equal halves. One one side, CH3CH2CO and on the
other side we have CH3CHClCO.
Hence the center is stereogenic. Also, since there are no elements of symmetry,
this molecule is definitely chiral!
Was this answer helpful ?
:
A
Look at the carbon where stereochemical data is given.
Now, we need to figure out if there are any elements of symmetry.
If we draw a plane that passes through this carbonand −H and −NH2,
we don't get two equal halves. One one side, CH3CH2CO and on the
other side we have CH3CHClCO.
Hence the center is stereogenic. Also, since there are no elements of symmetry,
this molecule is definitely chiral!
Was this answer helpful ?

















Submit Solution