12th Grade > Physics
X - RAYS MCQs
Total Questions : 12
| Page 2 of 2 pages
Answer: Option B. -> X-Rays are highly energetic, as compared to visible light.
:
B
X-rays are a part of electromagnetic spectrum and they fall in the high energy region of it.
They are not radioactive neither charged, but it is only them being highly energetic that they may affect the chemical bonds inside the body and change certain mechanisms if not destroy them (simply speaking creating mutation in fundamental building blocks of human body as D.N.A.). Medical science calls these types of mutations "Cancer".
That’s why they are harmful.
But don't get scared of becoming some mutilated character next time when you go for an x-ray examination!! The chances of this are very less and the benefits are definitely more (your doctor gets to see your fractures). The cancer may be caused only due to excess exposure to direct radiation.
:
B
X-rays are a part of electromagnetic spectrum and they fall in the high energy region of it.
They are not radioactive neither charged, but it is only them being highly energetic that they may affect the chemical bonds inside the body and change certain mechanisms if not destroy them (simply speaking creating mutation in fundamental building blocks of human body as D.N.A.). Medical science calls these types of mutations "Cancer".
That’s why they are harmful.
But don't get scared of becoming some mutilated character next time when you go for an x-ray examination!! The chances of this are very less and the benefits are definitely more (your doctor gets to see your fractures). The cancer may be caused only due to excess exposure to direct radiation.
Question 12. Figure shows the intensity-wavelength relations of X-rays coming from two different Coolidge tubes. The solid curve represents the relation for the tube A in which the potential difference between the target and the filament is VA and the atomic number of the target material is ZA. These quantities are VB and ZB for the other tube. Then,
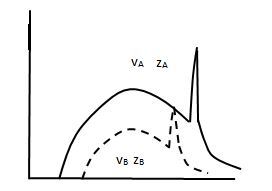

Answer: Option B. -> VA > VB, ZA < ZB
:
B
In the hint given we asked you to think about two questions:
1) How minimum wavelength is affected by accelerating potential and atomic number?
2) How wavelength of characteristic Kα x-ray is affected by accelerating potential?
Let's see whether answering these two leads us to our solution or not ?
Let's answer the 1st question first.
Minimum wavelength is given by λ0 = hceV0.
We see that it only depends on the accelerating potential but not the atomic number.
Also, a lesser λ0 implies to a higher accelerating potential, hence
VA > VB.
Now let's answer the 2nd question.
Kα refers to the transition of electron from L shell to K . In the process energy is released. If the Z is greater, then the force of attraction for this electron will be higher hence we can conclude that more energy will be released.
It’s also evident from the Moseley's law using which we see that the wavelength of Kα x - ray depends on atomic number but is not related to accelerating potential.
The relation is given by:
√v=a(Z−b)OR√cλ=a(Z−b)
We find that a lesser Kα Characteristic x-ray wavelength implies a higher atomic number.
From the graph;
ZA < ZB.
:
B
In the hint given we asked you to think about two questions:
1) How minimum wavelength is affected by accelerating potential and atomic number?
2) How wavelength of characteristic Kα x-ray is affected by accelerating potential?
Let's see whether answering these two leads us to our solution or not ?
Let's answer the 1st question first.
Minimum wavelength is given by λ0 = hceV0.
We see that it only depends on the accelerating potential but not the atomic number.
Also, a lesser λ0 implies to a higher accelerating potential, hence
VA > VB.
Now let's answer the 2nd question.
Kα refers to the transition of electron from L shell to K . In the process energy is released. If the Z is greater, then the force of attraction for this electron will be higher hence we can conclude that more energy will be released.
It’s also evident from the Moseley's law using which we see that the wavelength of Kα x - ray depends on atomic number but is not related to accelerating potential.
The relation is given by:
√v=a(Z−b)OR√cλ=a(Z−b)
We find that a lesser Kα Characteristic x-ray wavelength implies a higher atomic number.
From the graph;
ZA < ZB.
















