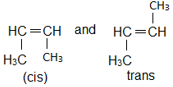12th Grade > Chemistry
ISOMERISM MCQs
Total Questions : 30
| Page 2 of 3 pages
Answer: Option B. -> Positional isomers
:
B
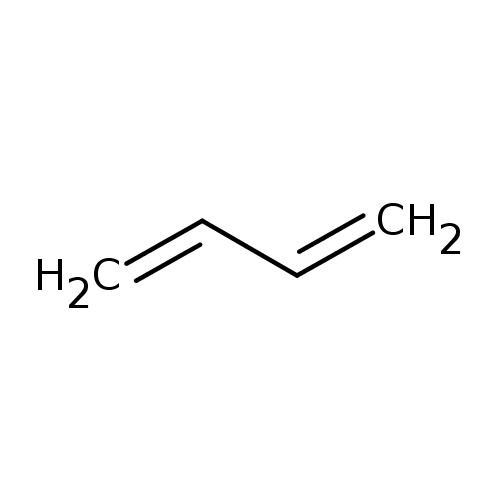
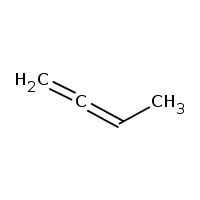
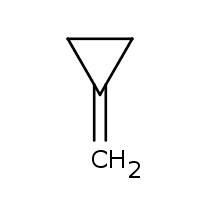
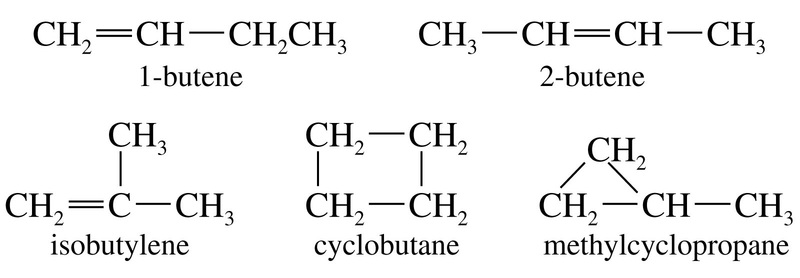
Positional isomersare constitutionalisomersthat have the same carbon skeleton and the same functional groups but differ from each other in the location of the functional groups on or in the carbonchain.
The two isomers differ in the position of the double bond. So they are positional isomers.
:
B
Positional isomersare constitutionalisomersthat have the same carbon skeleton and the same functional groups but differ from each other in the location of the functional groups on or in the carbonchain.
The two isomers differ in the position of the double bond. So they are positional isomers.
Answer: Option B. -> (CH3)3C−OH
:
B
Diethylether has a formula of C4H10O
If you count the atoms in the options, you will get this only in(CH3)3C−OH
:
B
Diethylether has a formula of C4H10O
If you count the atoms in the options, you will get this only in(CH3)3C−OH
Answer: Option D. -> None
:
D
They are not isomers as their molecular formula is different.
Molecular formula of acetaldehyde is C2H4O and of acetoneis C3H6O
:
D
They are not isomers as their molecular formula is different.
Molecular formula of acetaldehyde is C2H4O and of acetoneis C3H6O
Answer: Option A. -> 1-phenyl-2-butene
:
A
Only 1-phenyl-2-butene will exibit cis-trans isomerism
:
A
Only 1-phenyl-2-butene will exibit cis-trans isomerism
Answer: Option B. -> Functional isomerism
:
B
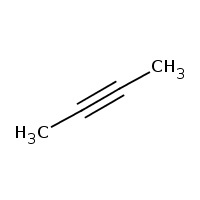
CH3−C≡C−CH3 is 2-butyne and CH2=CH−CH=CH2 is 1,3-butadiene.
As their molecular formula is the same but functional group is different they are functional isomers.
2-butyne has a triple bond while 1,3-butadiene has two double bonds.
:
B
CH3−C≡C−CH3 is 2-butyne and CH2=CH−CH=CH2 is 1,3-butadiene.
As their molecular formula is the same but functional group is different they are functional isomers.
2-butyne has a triple bond while 1,3-butadiene has two double bonds.
Answer: Option A. -> 2-butene
:
A
The compounds with each doubly bonded carbon attached to two different groups (like Cab=Cab, Cab=Ccd) exhibit geometrical isomerism i.e., cis and trans forms. The geometrical isomerism arises due to the restricted rotation of the double bond.
However, even though there is restricted rotation for a triple bond, alkynes do not exhibit geometrical isomerism, since the triply bonded carbons are attached to only one group each.
:
A
The compounds with each doubly bonded carbon attached to two different groups (like Cab=Cab, Cab=Ccd) exhibit geometrical isomerism i.e., cis and trans forms. The geometrical isomerism arises due to the restricted rotation of the double bond.
However, even though there is restricted rotation for a triple bond, alkynes do not exhibit geometrical isomerism, since the triply bonded carbons are attached to only one group each.
Answer: Option D. -> RCH2NO2
:
D
Nitro compound exhibit tautomerism RCH2NO2
:
D
Nitro compound exhibit tautomerism RCH2NO2